Credit: SWOG
PORTLAND, OR – For the second year, SWOG, the cancer clinical trials network, and its charity, The Hope Foundation, are providing $125,000 to five U.S. Department of Veterans Affairs medical centers to expand access to cancer clinical trials.
Under the VA Integration Support Program, medical centers receive $25,000 in seed funding to help them enroll veterans in trials run by SWOG and other members of the National Cancer Institute's National Clinical Trials Network (NCTN). This means more veterans can enroll in research studies featuring cutting-edge medicines. The publicly funded NCTN offers well over 200 open trials at any given time, trials testing prevention and treatment strategies for a variety of cancers, including lung, prostate, and colorectal cancers – the most common forms in veterans.
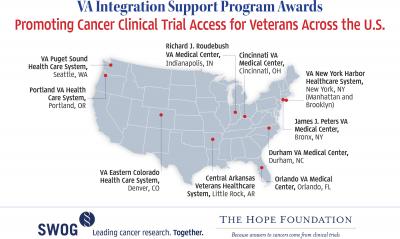
Winners of the VA Integration Support Program awards for 2016 are:
- James J. Peters VA Medical Center, Bronx, NY
- Orlando VA Medical Center, Orlando, FL
- Portland VA Health Care System, Portland, OR
- VA Puget Sound Health Care System, Seattle, WA
- Richard J. Roudebush VA Medical Center, Indianapolis, IN
Last year, SWOG and The Hope Foundation awarded funds to these VA centers:
- Central Arkansas Veterans Healthcare System, Little Rock, AR
- Cincinnati VA Medical Center, Cincinnati, OH
- Durham VA Medical Center, Durham, NC
- VA Eastern Colorado Health Care System, Denver, CO
- VA New York Harbor Healthcare System, Manhattan Campus, New York, NY
Clinical trials are an important option for any cancer patient managing their disease. Trials test new treatments, and are sometimes the only way to access immunotherapies or precision medicines. For many reasons, most stemming from a lack of time and money, veterans' ability to join trials sponsored by the National Cancer Institute and other groups has decreased dramatically.
SWOG and The Hope Foundation are working to turn that trend around, and their efforts are paying off. Winners of the 2015 VA Integration Support Program grants have expanded the hours of current research staff or hired new staff to process paperwork, screen patients, or collect tissue or other biological samples needed to take part in trials.
As a result, 13 NCTN trials are now open to veterans at these sites, including the landmark Lung-MAP precision medicine trial testing new drugs for squamous cell lung cancer. Soon, the NCI-MATCH trial will also be available at some sites. Veterans are responding to the increasing access. About 50 have been screened for trial participation, and 12 have enrolled.
One is Jerry Valentino, a lung cancer patient who joined SWOG's Lung-MAP trial through the VA Connecticut Healthcare System. Valentino, a 70-year-old Vietnam War veteran, has experienced no side effects from his trial drug and is responding well to treatment. Scans show his cancer is gone – and hasn't returned.
"Trials are the only way we know if drugs work or they don't," Valentino said. "It feels good to be part of the system that is moving medicine forward. This program is really helping veterans – and by all means they deserve it."
Dr. Charles Blanke, SWOG group chair, agrees. Blanke created the VA Integration Support Program to help veterans and support the NCTN. Groups in the NCTN – SWOG, Alliance for Clinical Trials in Oncology, ECOG-ACRIN Cancer Research Group, NRG Oncology, and Children's Oncology Group – are a major part of the nation's cancer research infrastructure and enroll tens of thousands of patients each year. All five groups are funded by the National Cancer Institute, and constitute the oldest and largest cancer research network in the nation.
"I am pleased with our progress and impact one year into this new program," Blanke said. "We are doing what we'd hoped to do – make great cancer trials available to veterans. Consideration of a clinical trial is a hallmark of excellent cancer care, and our veterans deserve the very best."
###
For information on the VA Integration Support Program, contact Morgan Cox at The Hope Foundation at (734) 998-6887 or [email protected].
SWOG is part of the National Cancer Institute's National Clinical Trials Network, the nation's oldest and largest cancer research network, and is a major part of the cancer research infrastructure in the U.S. and the world. SWOG has over 12,000 members in 46 states and six foreign countries who design and conduct cancer clinical trials to improve the lives of people with cancer. Founded in 1956, SWOG's 1,300 trials have led to the approval of 14 cancer drugs, changed more than 100 standards of cancer care, and saved more than 2 million years of human life. Learn more at swog.org.
The Hope Foundation is a public charity that supports SWOG's work by providing funds for research grants and fellowships, physician education, clinical trial support, and patient advocacy.
Media Contact
Wendy Lawton
[email protected]
503-348-8675
@SWOG
http://swog.org