In the rapidly evolving landscape of biomedical research, the interplay between mechanical signals and cellular behavior has become a pivotal area of investigation. A recent study by Huang, HN., Lee, LW., Kuo, CH., and colleagues explores this nexus, with specific attention to the regulation of the long non-coding RNA Neat1 and the protein PSPC1 in the context of renal progenitor cells driven by TGF-β1 signaling. This research unveils crucial insights into how substrate stiffness can dictate cellular responses, potentially influencing renal cell fate decisions.
At the heart of this study is the concept of mechanotransduction, the process through which cells convert mechanical stimuli from their environment into biochemical signals. This phenomenon has garnered increasing interest, particularly in the realm of stem cell biology, where local tissue stiffness can dramatically impact cell differentiation and function. The researchers hypothesize that variations in substrate stiffness can influence the expression levels of Neat1 and PSPC1, key players in the regulation of cellular behavior in renal progenitor cells.
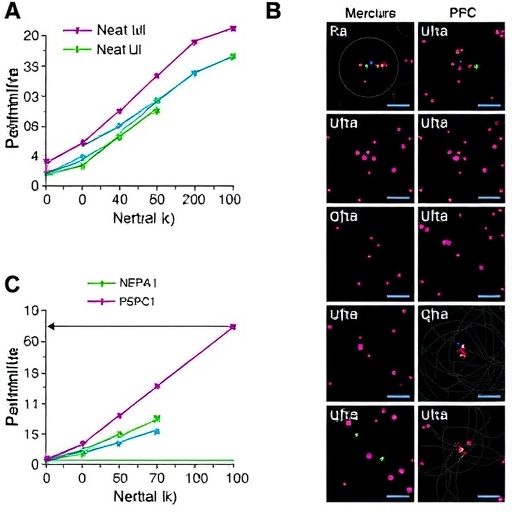
Utilizing advanced experimentation techniques, the authors manipulate the mechanical properties of the substrate on which the renal progenitor cells grow. By engineering surfaces with varying stiffness, they are able to probe how these physical properties affect cellular characteristics. The results illustrate that stiffer matrices promote renal progenitor cell differentiation, with significant implications for regenerative medicine practices aimed at kidney repair and regeneration.
Additionally, the study emphasizes the relevance of TGF-β1, a cytokine known for its roles in cellular differentiation, proliferation, and fibrosis. TGF-β1 is widely recognized for its key role in kidney disease progression, making it an important factor to study in the context of renal progenitor cells. The researchers find that in the presence of TGF-β1, stiff substrates upregulate the expression of Neat1 and PSPC1, further illuminating the intricate relationship between mechanical factors and cellular signaling pathways.
Neat1, a long non-coding RNA, has gained attention for its involvement in cellular stress responses and nuclear organization. Its upregulation in response to mechanical stiffness suggests a functional role in cellular adaptation to environmental cues. Similarly, PSPC1 is involved in RNA processing and stress granule dynamics, indicating that its regulation is equally crucial for renal progenitor cell fate. The synergistic interaction between these two molecular players highlights the complex regulatory networks that govern cell behavior.
The methodological rigor employed in this study allows for a nuanced understanding of the mechanistic underpinnings linking substrate stiffness to cellular outcomes. High-resolution imaging techniques, along with quantitative analyses, reveal that not only does stiffness influence the expression levels of Neat1 and PSPC1, but it also alters their spatial localization within the cells. This underscores the idea that mechanical cues can dictate not only how much of a molecule is produced, but where it operates within the cell.
The implications of these findings extend beyond basic science into the realm of clinical application. Understanding how renal progenitor cells respond to mechanical cues provides valuable insight for tissue engineering and regenerative therapies aimed at combating renal diseases. By modulating substrate stiffness in therapeutic contexts, it may be possible to direct cell fate towards desired outcomes, thereby enhancing the efficacy of stem cell-based interventions.
Moreover, as the global burden of kidney disease continues to rise, the urgency for innovative treatments intensifies. The study’s findings can inform the design of biomaterials suitable for renal tissue engineering, enabling the development of scaffolds that promote optimal cellular behavior. This would ensure that the engineered tissues develop functional characteristics akin to natural kidney tissue, paving the way for successful transplantation and integration.
In essence, the interplay of mechanical properties and cellular signaling presents a promising frontier in regenerative medicine. As this area of research matures, the integration of material science with cellular biology will likely yield further breakthroughs that facilitate the manipulation of cell fate in a more controlled manner. This study stands as a testament to the vibrant exploration of these themes, highlighting the potential for a greater understanding of kidney biology.
As we anticipate future advancements in this field, the role of researchers like Huang, HN., Lee, LW., and Kuo, CH. remains critical. Their work not only enriches our comprehension of renal progenitor cell biology but also opens avenues for innovative approaches in treating kidney disorders. Harnessing the principles of mechanotransduction may indeed lead to novel strategies that redefine how we approach tissue engineering and regenerative therapies.
Investigating the mechanistic details of how substrate stiffness influences renal progenitor cell behavior sets a foundation for future studies aimed at unraveling the complexities of cellular responses to their mechanical environment. As researchers continue to delineate these pathways, we are likely to witness a transformation in how we conceptualize and address kidney health and disease management.
In conclusion, the intersection of mechanical signaling and renal biology presents an exciting frontier that holds promise for advancing regenerative medicine. The work of Huang and collaborators not only elucidates important biological principles but also serves as a clarion call for the integration of physical and biological sciences in the quest to understand and treat kidney disease.
Subject of Research: Mechanotransduction in renal progenitor cells and its regulation through substrate stiffness.
Article Title: Regulation of the mechanoresponsive Neat1 and PSPC1 by substrate stiffness in TGF-β1-induced renal progenitor cell fate.
Article References:
Huang, HN., Lee, LW., Kuo, CH. et al. Regulation of the mechanoresponsive Neat1 and PSPC1 by substrate stiffness in TGF-β1-induced renal progenitor cell fate.
J Biomed Sci 32, 99 (2025). https://doi.org/10.1186/s12929-025-01196-w
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s12929-025-01196-w
Keywords: mechanotransduction, renal progenitor cells, substrate stiffness, Neat1, PSPC1, TGF-β1, regenerative medicine, kidney disease, tissue engineering.
Tags: advanced experimentation in biomedical researchcellular responses to environmental stiffnessengineering substrate properties for cell studiesimpact of mechanical signals on cell fatelong non-coding RNA in cell regulationmechanotransduction in biologyNeat1 regulation in renal cellsPSPC1 protein function in stem cellsrenal progenitor cell differentiationstem cell biology and mechanical cuessubstrate stiffness and cellular behaviorTGF-β1 signaling pathways