Actin filaments — protein structures critical to living movement from single cells to animals — have long been known to have polarity associated with their physical characteristics, with growing “barbed” and shrinking “pointed” ends. The ends of the filament are also different in the way they interact with other proteins in cells. However, the mechanism that determines these differences has never been entirely clear to scientists. Now, researchers from the Perelman School of Medicine at the University of Pennsylvania have revealed key atomic structures of the ends of the actin filament through the use of a technique called cryo-electron microscopy (cryo-EM). The study, published in Science, provides fundamental insights that may help fill in details behind disorders affecting some muscle, bone, heart, neurological, and immune disorders that are the result of actin defects or deficiencies.
Credit: Courtesy of Penn Medicine
Actin filaments — protein structures critical to living movement from single cells to animals — have long been known to have polarity associated with their physical characteristics, with growing “barbed” and shrinking “pointed” ends. The ends of the filament are also different in the way they interact with other proteins in cells. However, the mechanism that determines these differences has never been entirely clear to scientists. Now, researchers from the Perelman School of Medicine at the University of Pennsylvania have revealed key atomic structures of the ends of the actin filament through the use of a technique called cryo-electron microscopy (cryo-EM). The study, published in Science, provides fundamental insights that may help fill in details behind disorders affecting some muscle, bone, heart, neurological, and immune disorders that are the result of actin defects or deficiencies.
Actin is the most abundant protein inside the cells of higher organisms, such as animals. It serves as the building-block for long, thin structures called filaments, which provide key structural support as part of the cell “cytoskeleton,” the system that gives cells their shape and polarity. Rapid changes in actin filaments underlie key cellular events such as movement along surfaces, cell-to-cell contact, and cell division. Actin filaments also are major elements in muscle fibers.
“The results of our study provide a mechanistic understanding of a process we have known about for more than 40 years, referred to as filament treadmilling, and impacts how we view the cellular roles of actin in health and disease,” said the study senior author Roberto Dominguez, PhD, the William Maul Measey Presidential Professor of Physiology at Penn.
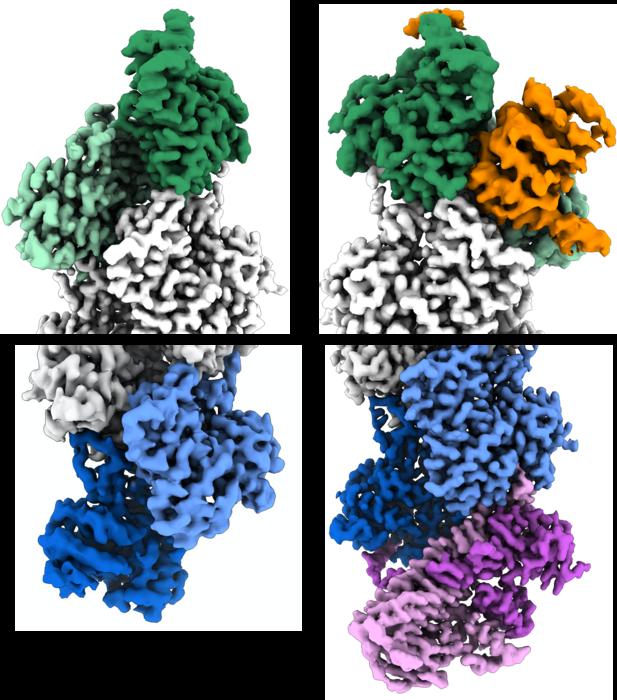
The dynamics of actin filaments are governed largely by the “treadmilling” process, through which individual actin proteins are shed from one filament end, known as the pointed end, and added at the other, barbed end. Actin filaments can be stabilized by distinct so-called “capping” proteins that bind to the filament ends to stop further addition or loss of individual actin proteins. Many other proteins also bind to the barbed and pointed ends of the actin filament. But the structural details determining the specificity of these interactions — the details that explain why these two ends function so differently — have been murky.
In their study, the researchers, including two Penn students — Peter Carman, PhD, a recent graduate student in Dominguez’s lab, and Kyle Barrie, PhD, a graduate student currently in the lab, who served as co-first authors — analyzed actin filaments using cryo-EM. With this high-resolution imaging technique, a researcher obtains many thousands of snapshots of a target molecule, aligns them computationally, and then averages them to reduce random image “noise”— yielding a 3-D reconstruction of the molecule that may be sharp enough to visualize individual atoms.
With artificial intelligence (AI) assistance, the researchers were able to focus on the ends of the filaments instead of their middle, as had previously been the norm in similar research. By doing so, they identified hundreds of thousands of filament end views, allowing them to obtain near-atomic scale reconstructions. These revealed a “flat” actin shape, or conformation, at the uncapped barbed end, versus a “twisted” conformation at the uncapped pointed end.
The data also detailed the structural changes induced by two actin filament-capping proteins, CapZ at the barbed end and tropomodulin at the pointed end. These are the two proteins found at the ends of the filament in skeletal and cardiac muscles, playing an essential role in the stabilization of actin filaments in muscle fibers, and, without these proteins, our muscles would fall apart.
Results from this study provide crucial mechanistic details for a deeper understanding of actin biology as a whole. The researchers believe these study insights should also be helpful in understanding and ultimately treating disorders caused by actin dysfunction.
Funding was provided by the National Institutes of Health (R01 GM073791, F31 HL156431).
Journal
Science
DOI
10.1126/science.adg6812
Method of Research
Observational study
Subject of Research
Cells
Article Title
Structures of the free and capped ends of the actin filament
Article Publication Date
25-May-2023




