Damages to the central nervous system (CNS), for example in the case of spinal cord injury, can result in permanent loss of sensory and motor function. It is because the severed axons are unable to regenerate. As of today, there are very limited options to help these patients regain their motor abilities. Scientists have been exploring ways to enable the regeneration of severed axons, with a view to developing viable treatments in the long term.
Credit: The Hong Kong University of Science and Technology
Damages to the central nervous system (CNS), for example in the case of spinal cord injury, can result in permanent loss of sensory and motor function. It is because the severed axons are unable to regenerate. As of today, there are very limited options to help these patients regain their motor abilities. Scientists have been exploring ways to enable the regeneration of severed axons, with a view to developing viable treatments in the long term.
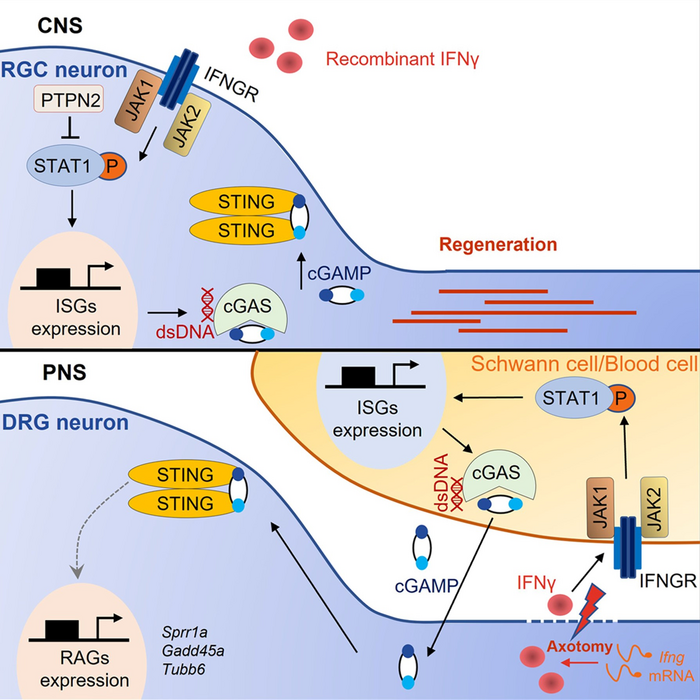
In a study using mice, a research team led by Cheng Associate Professor Kai LIU of the Division of Life Science, the Hong Kong University of Science and Technology (HKUST), untangled some of the complexities in the regeneration of severed axons. They found that the deletion of PTPN2, a phosphatase-coding gene, in neurons can prompt axons to regrow. When combined with the type II interferon IFNγ, it can further accelerate the process and boost the number of axons regenerated. The results have recently been published in the scientific journal Neuron.
The human nervous system is composed of two parts, namely the central and peripheral nervous systems. Unlike the central nervous system, peripheral nerves have stronger ability to regrow and repair by themselves after injury. Scientists have yet to fully understand the relationship between this self-repair and the intrinsic immune mechanism of the nervous system. Two mysteries the team wanted to resolve were how immune-related signaling pathways affected neurons after injury, and whether they could enhance axonal regeneration directly.
This study investigated whether the signaling pathway IFNγ-cGAS-STING had any role in the regeneration process of peripheral nerves. Researchers found that peripheral axons could directly modulate the immune response in their injured environment to promote self-repair after injury.
In previous research, Prof. Liu’s team had already demonstrated that elevating the neuronal activity and regulating the neuronal glycerolipid metabolism pathway could boost axon regenerative capacity. The current study is providing further insights into the search of treatment solutions for challenging conditions such as spinal cord injuries, with one possible option being the joining of several types of different signaling pathways.
The co-first authors of this project are Drs. Xu WANG and Chao YANG of HKUST. The work was done in collaboration with Prof. Zhong-Yin ZHANG of Purdue University alongside Profs. Ruohao WU, and Peiyuan QIAN, Jiguang WANG of HKUST.
Journal
Neuron
Article Title
Driving axon regeneration by orchestrating neuronal and non-neuronal innate immune responses via the IFNγ-cGAS-STING axis
Article Publication Date
11-Nov-2022




