A recent study conducted by researchers at Nagoya University in Japan has revealed critical insights into the neurological decline associated with aging, specifically through experiments conducted on the nematode Caenorhabditis elegans. Unlike previous beliefs that attributed age-related cognitive decline to decreased neuronal activity, this research suggests that excessive activation of certain neurons over time is a significant contributor to the deterioration of brain function. The findings, published in the prestigious Proceedings of the National Academy of Sciences, open up new avenues for potential interventions, including dietary changes that could help mitigate cognitive decline associated with aging.
Understanding how the brain operates during aging has become a vital area of study in neuroscience. Traditionally, scientists believed that as organisms age, their neurons gradually lost efficacy and activity, leading to cognitive impairments. However, the current study challenges this notion by uncovering the role of hyperactivation in specific neuronal types within the nematodes. The study focuses on a particular behavior known as thermotaxis, where C. elegans can learn to associate particular temperatures with the presence of food. This behavior is crucial for their survival, and understanding its decline provides valuable insights into the mechanisms of aging.
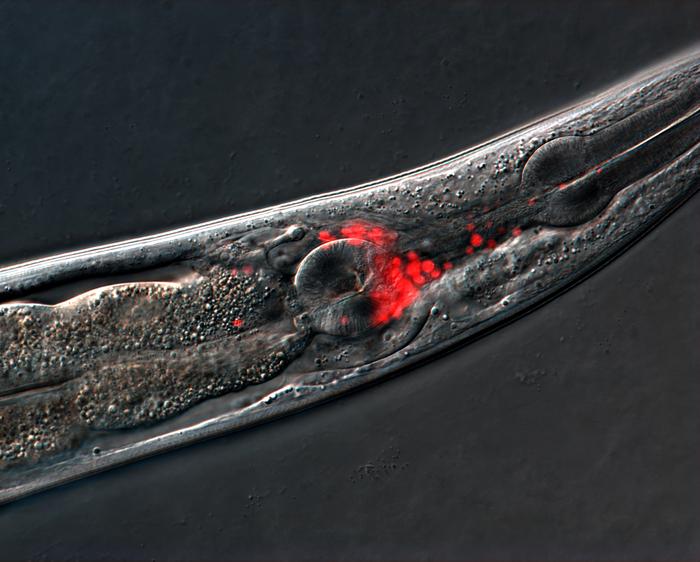
C. elegans, a microscopic roundworm, serves as an ideal model organism for studying neurobiology due to its simplicity; it comprises a mere 302 neurons, yet its neurological processes show significant parallels to those of humans. In their research, the team meticulously tracked the brain function of these nematodes as they aged, observing how connections between neurons shifted over time. Interestingly, instead of observing diminished neuronal activity, researchers found that certain sensory neurons became hyperactive, leading to confusion in their behavioral responses to environmental cues.
Associate Professor Kentaro Noma, who led the research, emphasized the importance of these findings: “Our work suggests that the acknowledged age-related decline in cognitive functions may be more deeply rooted in neuronal hyperactivation than previously thought. This hyperactivation disrupts the normal neuronal networks, ultimately impairing the organism’s behavioral responses.” The data collected through their experimental study provides a paradigm shift in how researchers may approach understanding and potentially treating cognitive decline.
The experiments illustrated a compelling narrative regarding the sensory neurons responsible for the thermotaxis behavior in C. elegans. Researchers found that the AFD sensory neurons and AIY interneurons, both essential for associative learning, exhibited almost no change in activity with age. This was surprising, as one would typically expect a decline in all components underpinning cognitive functions. Instead, the hyperactivity of sensory neurons AWC and AIA was discovered to stray from the norm, leading to behavioral decline in older worms.
To delve deeper into the complexities of these findings, the researchers conducted a series of elimination experiments, where they selectively removed specific neuron types from the nematode brain. Astonishingly, even after the removal of the AWC or AIA neurons—previously thought essential for navigating toward favorable temperature—C. elegans still demonstrated the ability to move toward the 23-degree location. This observation raised pivotal questions about the redundancy and compensation mechanisms present in neuronal networks that allow for continued function despite the loss of certain components.
The investigation into aged nematodes revealed compelling evidence that the spontaneous hyperactivation of AWC and AIA occurred alongside the animals’ decline in behavioral aptitude. By using various techniques to measure neuronal activities, the researchers established a clear causal relationship between excessive neuronal firing and the inability of the worms to properly execute learned behaviors. The pivotal takeaway from this aspect of the research underscores the necessity of maintaining balanced neuronal activity as a potential mechanism to fend off the cognitive ravages of aging.
In pursuit of interventions, the researchers discovered that altering the dietary sources of the aged nematodes could effectively suppress the hyperactivation of specific neurons. This led to the suggestion that dietary modifications could play a crucial role in maintaining healthy brain function as an organism ages. “Changing the type of bacteria in the diets of C. elegans enabled us to curb neuronal hyperactivation,” Noma recounted. “This opens exciting possibilities for humans; lifestyle and dietary shifts may similarly influence neurological health.”
The implications of this research resonate beyond the realm of C. elegans, beckoning a broader application to human cognitive health. While the model organism presents a simplified version of the complexities associated with the human brain, the overlapping genetic and mechanistic elements suggest potential transferable insights into human aging processes. Understanding the balance of neuronal activities and their interactions could yield new therapeutic strategies targeting hyperactivation in aging brains.
Through continued inquiry, the researchers advocate for a paradigm shift in the understanding of brain aging. As Noma articulated, “By directing attention toward neuronal hyperactivation, rather than merely the decline, we can uncover new strategies to enhance cognitive function in aging populations. Our ongoing research will strive to elucidate effective methods to modulate neuronal hyperactivity.”
As the field of neuroscience evolves, studies like these guard the potential for innovative therapies informed by the discoveries made in simpler organisms. This latest research embodies the resilience of science in the quest to combat the multifaceted challenges posed by an aging population, reaffirming the necessity for holistic approaches to promoting cognitive longevity.
The findings from Nagoya University thus serve not only as a scientific milestone but as a call to action for broader dietary research targeting neurological health. Continued exploration into the mechanisms of neuronal behavior, coupled with comprehensive lifestyle assessments, can enrich our understanding of how to preserve cognitive function throughout life.
In summary, this groundbreaking study signifies a remarkable advancement in our comprehension of age-related cognitive decline, pivoting the focus from merely decreasing neuronal activity to the critical implications of neuron hyperactivity. The research establishes a foundation for new strategies aimed at fortifying brain health as we encounter the inevitable changes that come with aging—a pursuit both vital and urgent in contemporary scientific discourse.
Subject of Research: Nematode model organisms; neuronal hyperactivation and aging
Article Title: Aberrant neuronal hyperactivation causes an age-dependent behavioral decline in Caenorhabditis elegans
News Publication Date: 7-Jan-2025
Web References: DOI: 10.1073/pnas.2412391122
References: Proceedings of the National Academy of Sciences
Image Credits: Credit: Kentaro Noma
Keywords: Cognitive function, Human brain, Sensory neurons, Worms, Neural networks, Ethology, Animal research.
Tags: aging and brain functionCaenorhabditis elegans researchcognitive decline in elderlydietary changes for cognitive healthexcessive neuronal activity effectshyperactivated neurons and aginginsights from nematode studiesmechanisms of cognitive impairmentneurological decline and interventionsneuroscience of agingPNAS research on agingthermotaxis behavior in nematodes