Credit: Michigan Medicine
Researchers are working to determine why the aortic valve doesn't form correctly in patients with the most common congenital heart defect: bicuspid aortic valve.
In a new Nature Communications study, the Michigan Medicine-led group found two genetic variants associated with the condition.
Bicuspid aortic valve is moderately heritable, yet experts are still figuring out which part of our DNA code explains why some BAV patients inherit the disease.
"We've completed the first successful genomewide study of bicuspid aortic valve, by studying subjects at U-M's Frankel Cardiovascular Center," says first author Bo Yang, M.D., Ph.D., a Michigan Medicine cardiac surgeon. "We are using state-of-the-art technology of induced stem cell and gene editing to dissect the genomic region we found to be associated with BAV. It's a great collaboration that will accelerate our scientific understanding of this disease."
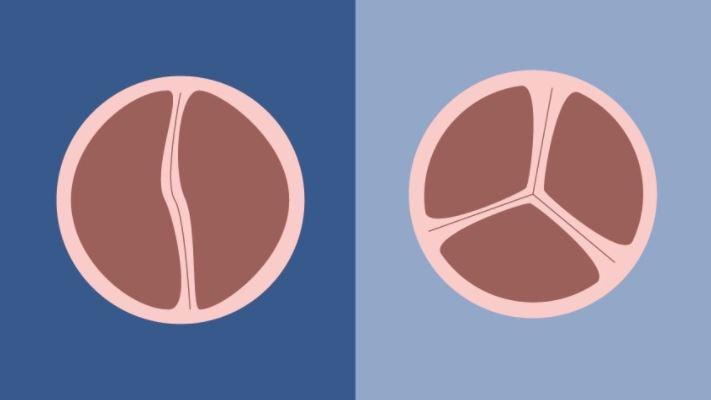
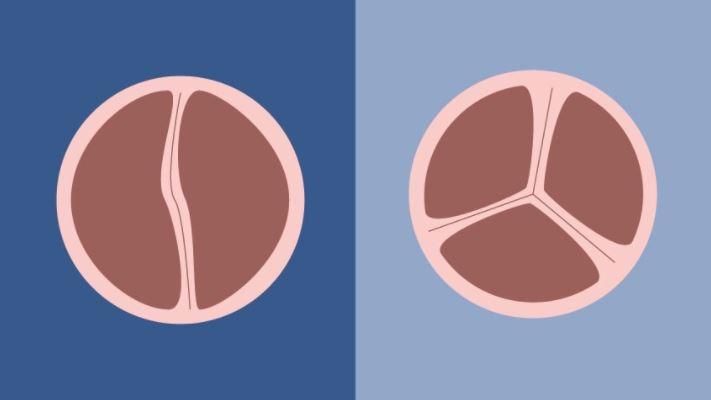
BAV patients have aortic valves with only two leaflets, rather than three, limiting the valve's function as the heart pumps oxygen-rich blood toward the aorta to enrich the body. The condition is associated with various complications, including a narrowed valve (aortic stenosis), a leaky valve (aortic insufficiency or regurgitation), an infection of the valve or an aortic aneurysm.
'A great head start'
The researchers performed genomewide association scans of 466 BAV cases from the Frankel Cardiovascular Center and 4,660 controls from the Michigan Genomics Initiative, with replication on 1,326 cases and 8,103 controls from collaborators at other leading institutions. They also reprogrammed the matured white blood cells to change them back into immortal cells (stem cells) and changed the genetic code of those cells to study the function of the variants they identified through the genomewide association study.
The team reports two genetic variants, both affecting a key cardiac transcription factor called GATA4, reached or nearly reached genomewide significance in BAV. GATA4 is a protein important to cardiovascular development in the womb, and GATA4 mutations have been associated with other cardiovascular defects.
"One of the regions we identify actually changes the protein coded by the gene, and the other likely changes expression levels of GATA4 during valve formation," says senior author Cristen Willer, Ph.D., professor of internal medicine, human genetics and computational medicine and bioinformatics. "Because most genetic variants associated with human disease are in the 99 percent of the genome that doesn't code for proteins, this finding gives us a great head start toward understanding the mechanism of how a genetic change outside the protein-coding part of the genome can lead to disease."
Specifically, the authors point to a disruption during the endothelial-mesenchymal transition, which is a critical step in the development of the aortic valve. Willer and Yang say this study, with support from the Frankel CVC and the Bob and Ann Aikens Aortic Program, adds new knowledge about the mechanism of BAV formation. They plan to continue to study the biological effect of both variants associated BAV in cells and animal models.
###
Collaborators from a variety of institutions provided replication of the result, including Harvard Medical School, the University of Texas, the Montreal Heart Institute, the Karolinska Institute and Icahn School of Medicine at Mount Sinai.
Media Contact
Haley Otman
[email protected]
734-764-2220
@umichmedicine
http://www.med.umich.edu
############
Story Source: Materials provided by Scienmag