Since COVID-19 emerged, scientists worldwide have sequenced more than 6 million genomes of SARS-CoV-2, the coronavirus behind the disease. Today, the number of SARS-CoV-2 genome sequences exceeds the total number of all other viral genomes.
Credit: National Institute for Communicable Diseases Control and Prevention, China.
Since COVID-19 emerged, scientists worldwide have sequenced more than 6 million genomes of SARS-CoV-2, the coronavirus behind the disease. Today, the number of SARS-CoV-2 genome sequences exceeds the total number of all other viral genomes.
Genomes are valuable because they provide a record of the evolution of a virus in a human host, along with information on the emergence of mutations. In a study, published in KeAi’s Journal of Biosafety and Biosecurity, a team of researchers analysed 2.8 million of the sequenced SARS-CoV-2 genomes and used the results to compile a ‘mutations blacklist’ of virus weak spots, and a ‘whitelist’ of mutations that make SARS-CoV-2 more transmissible. In addition, the team has developed an online SARS-CoV-2 mutation and variant monitoring and pre-warning system (MVMPS).
Professor Jianguo Xu of China’s National Institute for Communicable Diseases Control and Prevention led the study. Reflecting on the findings, he explained: “We found that six types of gene-level mutations had reached saturation in the sequenced genome population we examined – when you reach saturation, it means that no novel mutations should appear in the future, even if more genomes are sequenced. This result points to most subsequent variants of SARS-CoV-2 being a combination of existing high-frequency mutations, rather than a newly-emerged mutation. For example, the variant BA.5 has a L452R mutation that escapes host immunity. Although this mutation appears in the Omicron variant, it was also found in the Delta variant. This kind of information is crucial, as it suggests these mutations might have a negative effect on viral survival, replication and transmission, information that can aid vaccine and drug development research.” These mutations were used to compile the team’s SARS-CoV-2 ‘mutations blacklist’.
He continued: “Some mutations can affect the virus transmission rate. We identified a total of 185 mutations that are significantly positively correlated with viral transmission, information that scientists can use to evaluate the transmissibility of new variants.” These findings were used to create the team’s SARS-CoV-2 ‘mutations whitelist’.
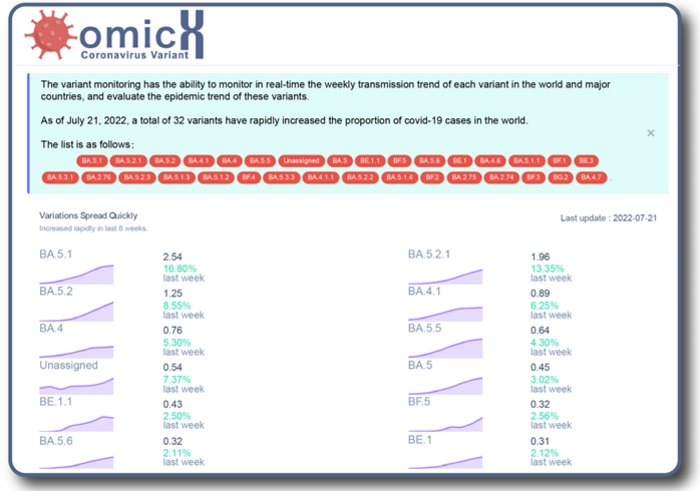
In addition, the team has built an online alert system – the MVMPS – which is home to a dynamic curve that shows, in real-time, proportions of each mutation and variant in all the SARS-CoV-2 strains. According to Prof. Xu, the hope is that the MVMPS will not only monitor the occurrence and development of these mutations and variants, but provide vital early warning information to help with prevention and control measures.
###
Contact the corresponding author: Jianguo Xu, [email protected]
The publisher KeAi was established by Elsevier and China Science Publishing & Media Ltd to unfold quality research globally. In 2013, our focus shifted to open access publishing. We now proudly publish more than 100 world-class, open access, English language journals, spanning all scientific disciplines. Many of these are titles we publish in partnership with prestigious societies and academic institutions, such as the National Natural Science Foundation of China (NSFC).
Journal
Journal of Biosafety and Biosecurity
DOI
10.1016/j.jobb.2022.06.006
Method of Research
Data/statistical analysis
Subject of Research
Not applicable
Article Title
“Mutation blacklist” and “mutation whitelist” of SARS-CoV-2
COI Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.




