An article published in The Faseb Journal describes a Brazilian study analyzing the correlation between two key energy metabolism regulation processes: the absorption and release of calcium ions by mitochondria, the organelles that generate energy for cells; and autophagy induced by calorie restriction. Autophagy occurs when cells break down and reuse their own cytoplasm.
Credit: Vitor de Miranda Ramos
An article published in The Faseb Journal describes a Brazilian study analyzing the correlation between two key energy metabolism regulation processes: the absorption and release of calcium ions by mitochondria, the organelles that generate energy for cells; and autophagy induced by calorie restriction. Autophagy occurs when cells break down and reuse their own cytoplasm.
The study was conducted at the Center for Research on Redox Processes in Biomedicine (Redoxome), a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP and hosted by the University of São Paulo’s Institute of Chemistry (IQ-USP).
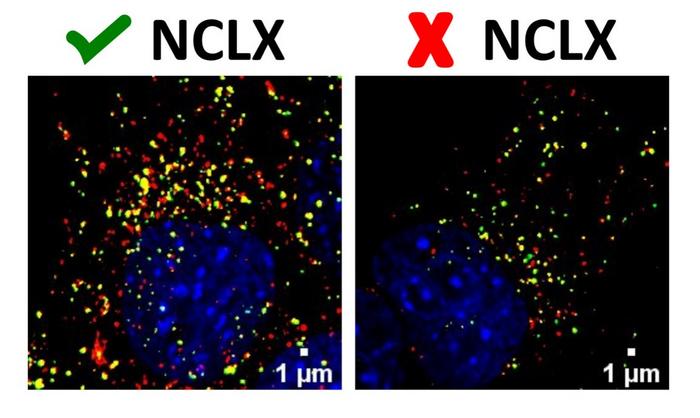
Redoxome’s researchers, led by Alicia Kowaltowski, a professor at IQ-USP, showed that the protein NCLX, the transporter responsible for calcium ion efflux, is a key regulatory node integrating mitochondria, autophagy control by calcium ions and cellular responses to nutrient availability, thereby establishing the link between autophagy and mitochondrial calcium.
“Our findings are important because of this link between processes. Both mitochondria and autophagy are involved in metabolism, so it makes sense that they’re connected. Researchers in cellular biology tend to see things separately, but we should bear in mind that in cells one process depends on and regulates the other. Although the study was fundamental science, which may seem somewhat abstract, this knowledge will certainly be useful to help develop targets for the treatment of various diseases,” said Vitor de Miranda Ramos, a member of the Redoxome team and first author of the article.
Ramos conducted part of the study while working in Viktor Korolchuk’s laboratory at Newcastle University in the United Kingdom, with support from FAPESP via a research internship abroad (BEPE).
As well as producing energy for cells, mitochondria participate directly in several calcium-sensitive cellular regulation pathways, thanks to their capacity to absorb and release calcium ions. Mitochondrial uptake of calcium ions is mediated by the mitochondrial calcium ion uniporter complex. NCLX, the mitochondrial Na+/Li+/Ca2+ exchanger, releases calcium ions from the mitochondrial matrix into the space between the inner and outer membranes in exchange for sodium ions.
Calcium affects almost all aspects of cellular life. Calcium ions are well-known second messengers in metabolic signaling and play an important role in the regulation of autophagy.
Cell recycling
Autophagy, a process highly conserved by evolution, consists of the degradation and recycling of cell components with the primary aim of maintaining cellular homeostasis. Because it removes unwanted elements and promotes nutrient availability, it is necessary for quality control, tissue renewal and metabolic regulation.
In addition to its basic functions, autophagy is activated in response to a decrease in nutrient availability and is considered one of the mechanisms responsible for the benefits of calorie restriction.
In the study, as a starting point for their investigation of the link between mitochondrial calcium transport and autophagy, the researchers submitted mice to four months of calorie restriction and found their liver mitochondria to contain higher levels of NCLX than samples from mice fed an unrestricted diet. They then used cultured liver cells to create models that mimicked calorie restriction and again observed an increase in NCLX expression.
Mechanism
After making measurements at different stages of the autophagy process, the researchers found that NCLX activity affected the initial steps in the process. In autophagy, cell components are sequestered by autophagosomes (vesicles that engulf the material to be degraded) and these in turn fuse with lysosomes to promote the digestion of organelles or proteins. The data obtained in the study led the researchers to conclude that alterations in intracellular calcium resulting from NCLX inactivation interfered with autophagosome formation, impairing the initial steps of autophagy.
Contrary to expectations, the researchers also found that NCLX inhibition did not affect mitochondrial production of adenosine triphosphate (ATP, the molecule that provides energy for cells) or AMP-activated protein kinase (AMPK), which increases when cell energy slumps.
“Regulation of autophagy by NCLX takes place via cellular calcium independently from the AMPK pathway,” Kowaltowski said.
According to the authors, more research is needed to elucidate the complex mechanisms of autophagy control by cytoplasmic calcium. “Calcium signaling is evidently very complex, and possibly ubiquitous in the cell: many proteins can bind to and be regulated by calcium, so it’s difficult to arrive at a specific mechanism,” Ramos said.
The brain uses a great deal of energy and needs many mitochondria. Accumulating cell damage can lead to cell death and the development of pathologies. “While we didn’t look specifically at pathological contexts, the link between calcium signaling and autophagy is interesting because it may relate to disease progression. When this transporter’s activity is lost, considerable damage ensues, and the process of clearing up this damage via autophagy is impaired. Future research will be able to clarify this further,” Kowaltowski said.
Journal
The FASEB Journal
DOI
10.1096/fj.202301368RR
Article Title
Mitochondrial sodium/calcium exchanger (NCLX) regulates basal and starvation-induced autophagy through calcium signaling
Article Publication Date
5-Feb-2024




