Credit: Volkov et al., Science 2017
Optogenetics enables specific nerve cells to be turned on and off using special light-sensitive 'protein switches'. One of the most important of these switches is Channelrhodopsin 2, the first 'light switch protein' to have been successfully expressed in nerve cells. Today it is used in laboratories worldwide, having played a key role in launching the field of optogenetics, an indispensable technique in neuroscience. Channelrhodopsin 2 is used in basic research in the fields of neuroscience, muscle physiology, and cell biology basic and could one day also find its way into medicine in the form of gene therapy. Researchers from Jülich, Frankfurt, Grenoble and Moscow have now discovered the structure of this light-sensitive protein.
With optogenetics, researchers can use light to control the activity of neurons with greater accuracy than ever before. The key to this technique is specific microbial rhodopsin-like proteins. When these proteins are inserted into the membrane of nerve cells, they act as light-sensitive switches, which activate the cells by transporting positive ions into them. If these ions are transported back out of the cell by another rhodopsin protein, the cell is deactivated. What colour of light the proteins react to and which particles they transport into or out of the cell varies from molecule to molecule.
This allows researchers to switch nerve cells on and off remotely using light pulses, enabling them to study how the cells work. In this way they can study interactions between neuronal circuits in cell cultures and in living animals much more precisely than was previously possible. In addition, this can be used to study neurodegenerative diseases much more accurately and develop potential treatments.
From structure to medical application
One of the most important of these switches is Channelrhodopsin 2, which comes from a single-celled freshwater algae. Until now, the high-resolution structure and the structural mechanism of this ion channel were not known. "We wanted to determine the molecular structure to work out the details of how this molecule works. This knowledge could allow improved applications, perhaps even medical applications," says Ernst Bamberg from the Max Planck Institute of Biophysics in Frankfurt. The hope is that the research will allow the development of improved optogenetic tools for research on neurodegenerative and muscular diseases, such as age-related macular degeneration or hearing loss, or for light-stimulated pacemakers for heart muscle synchronisation. "Initial clinical trials with a Channelrhodopsin 2-based gene therapy for age-related macular degeneration have already been performed," notes Bamberg.
Structural biologist Valentin Gordeliy, his colleagues at ICS-6 in Jülich, and researchers from Grenoble, Moscow and Frankfurt have made significant progress in answering these questions. "We have been able to determine the structure of channelrhodopsin, thereby establishing the molecular basis to understand how the switch works in detail", says Gordeliy.
Ion channel with three gates
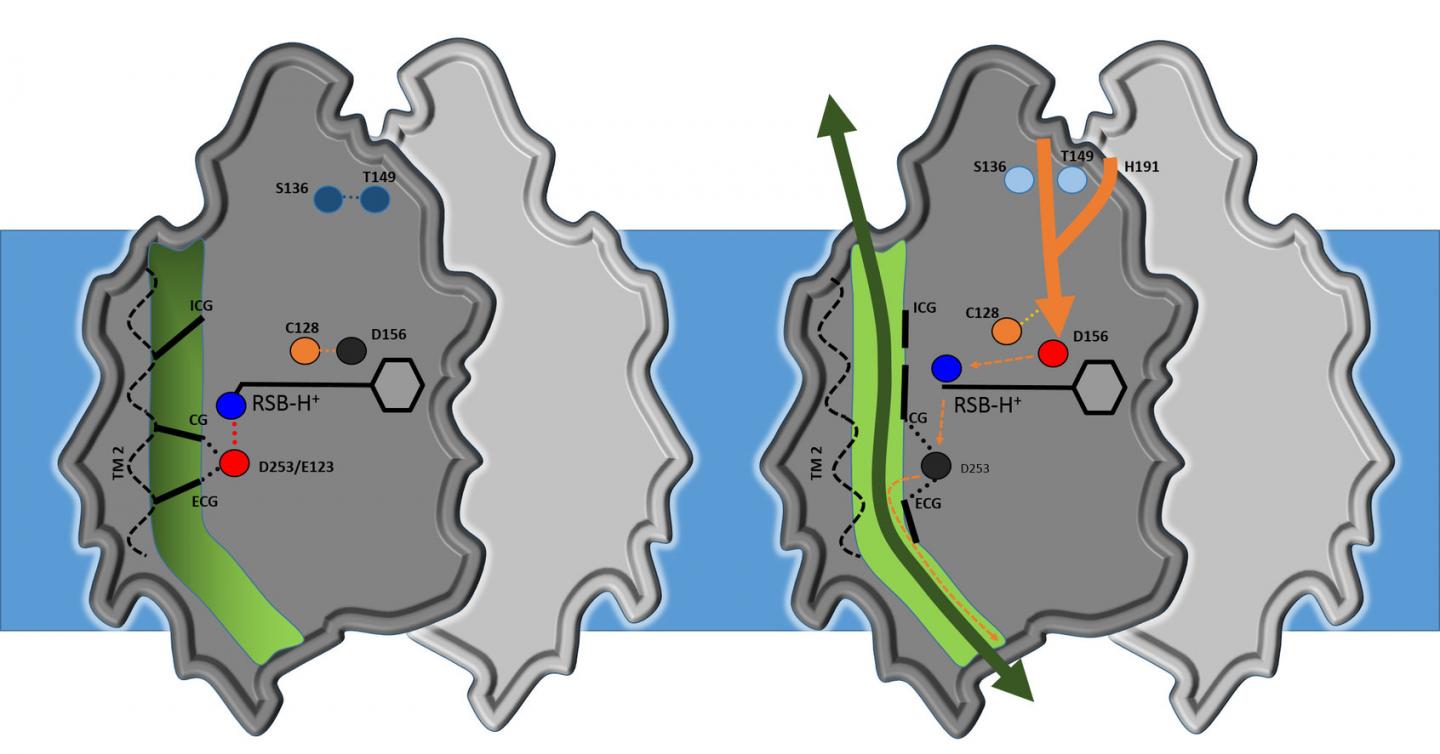
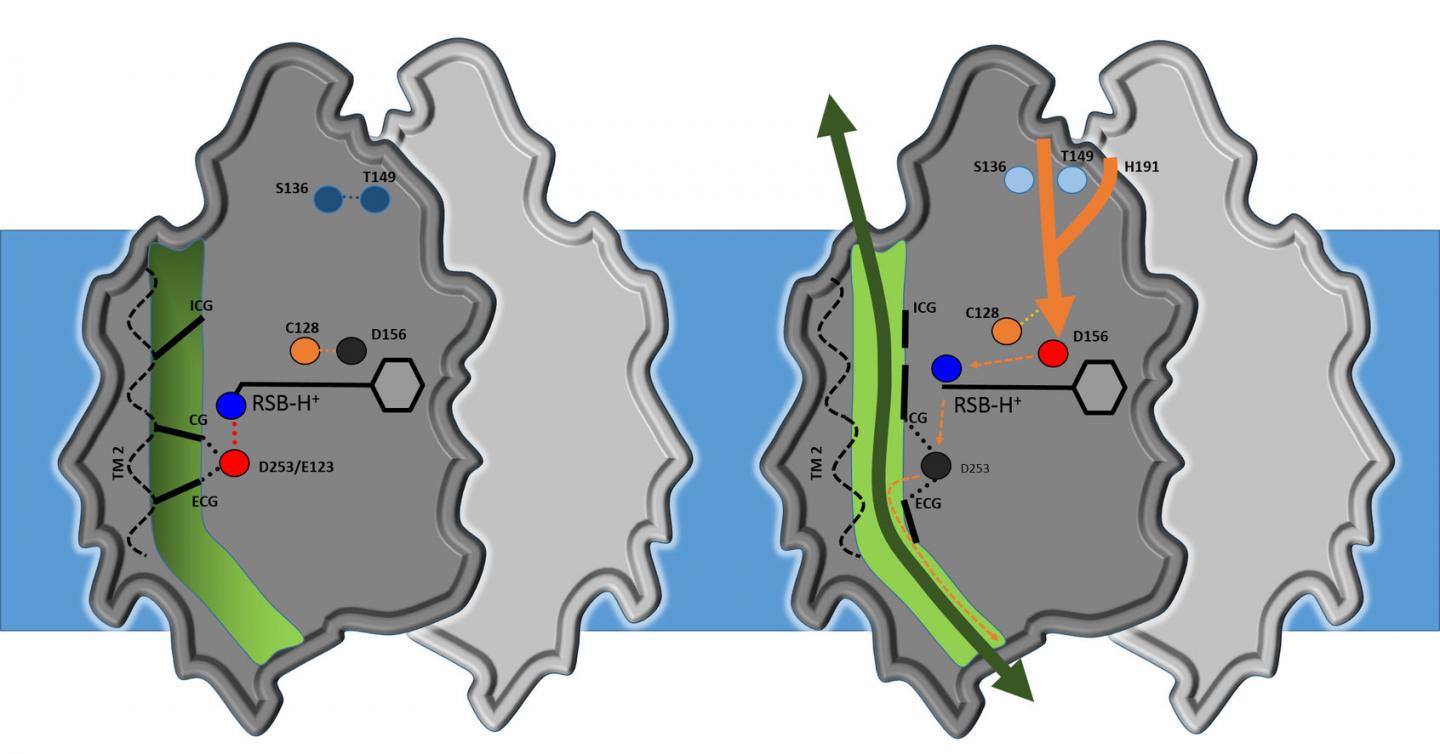
Analysis shows that the core structure of channelrhodopsin consists of four cavities separated by three flexible constrictions which act as gates. Highly simplified, it works as follows: when it's dark, the gates are closed; when the researchers turn the light on, all three gates open, allowing ions to flow through. This activates the nerve cell. Water molecules act as gatekeepers, forming a chain of hydrogen bonds from water molecule to water molecule. When channelrhodopsin is exposed to blue light, the retinal pigment on the upper side of the protein gives the order for the gate to open. The chain of water molecules then breaks, causing the three gates to open and producing a pore extending right through the protein.
"Our results are a milestone in optogenetics," says Gordeliy, "as they open up the possibility of constructing new channels with specific properties." According to Institute Director Dieter Willbold: "These results are an excellent example of the strong links between structural biology and brain research. That's why, in addition to our focus on neurodegenerative disorders, we have long pursued research on optogenetics. This is an area that really showcases the benefits of combining the Institute's structural biology methods and expertise with research on neurodegenerative disorders and treatments."
###
Original Publication
Oleksandr Volkov, Kirill Kovalev, Vitaly Polovinkin, Valentin Borshchevskiy, Christian Bamann, Roman Astashkin, Egor Marin, Alexander Popov, Taras Balandin, Dieter Willbold, Georg Büldt, Ernst Bamberg, Valentin Gordeliy
Structural insights into ion conduction by channelrhodopsin 2
Science; 24 November, 2017
Media Contact
Dr. Ernst Bamberg
[email protected]
49-696-303-2000
@maxplanckpress
http://www.mpg.de
Original Source
https://www.mpg.de/11814934/channelrhodopsin-structure http://dx.doi.org/10.1126/science.aan8862