Study describes new mechanism for terminating transcription of DNA into RNA in bacteria
Credit: Babitzke Laboratory and Dani Zemba, Penn State
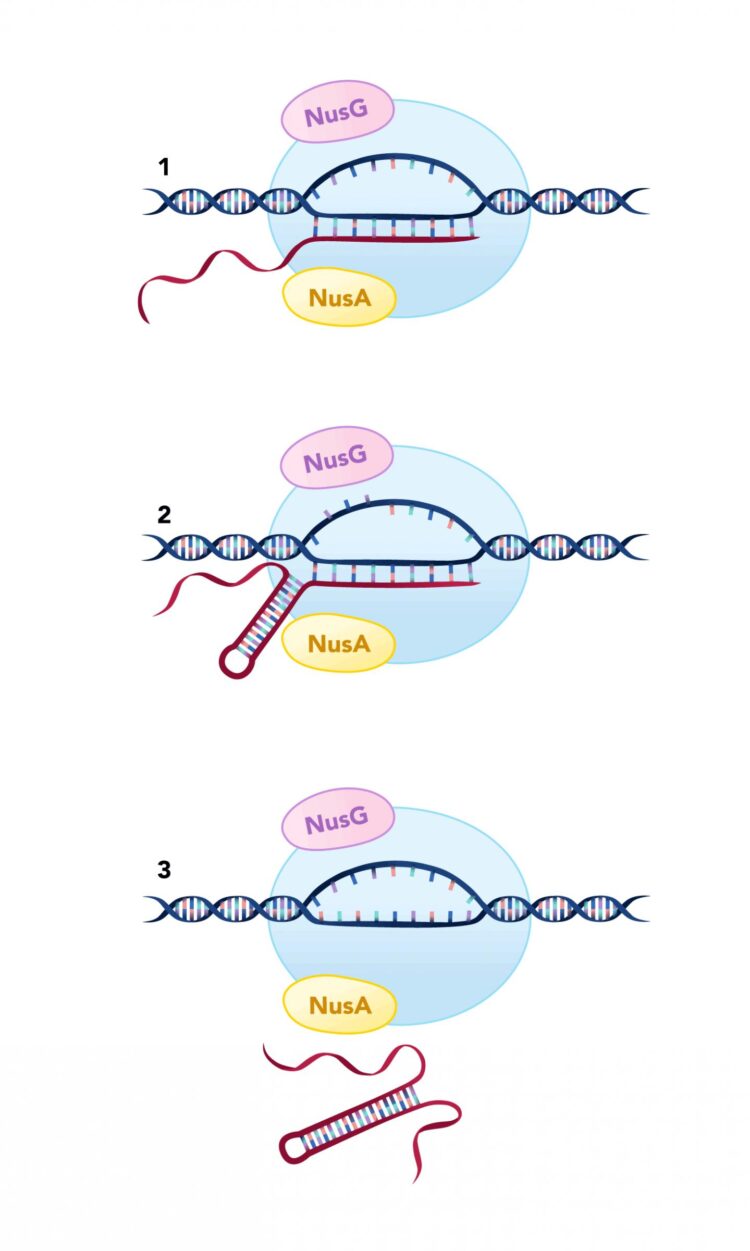
The protein, known as NusG, pauses the transcription machinery at specific DNA sequences to facilitate what is called “intrinsic termination” and prevent unwanted transcription that could disrupt cellular function.
A new study, led by Penn State researchers, shows that NusG and the related protein, NusA, together facilitate termination at about 88% of the intrinsic terminators in the bacteria Bacillus subtilis. Understanding this process expands our basic knowledge of this key cellular function and could eventually aid in the development of antibiotics that target and disrupt gene regulation in bacteria. A paper describing the research appears online in the journal eLife.
“For a cell to access the genetic information stored in DNA, it first must be transcribed into RNA by an enzyme called RNA polymerase,” said Zachary F. Mandell, a graduate student at Penn State and first author of the paper. “This process is highly coordinated to ensure that the right genes are expressed at the right times and at appropriate levels for the cell to function properly. We are interested in understanding the mechanisms that allow the cell to stop transcription at precise locations along the genome.”
Proper regulation of gene expression occurs in three basic stages. First, transcription is initiated by RNA polymerase that binds to the DNA at the beginning of the sequence that is being transcribed. NusA and NusG then bind to RNA polymerase during the elongation process to make an RNA copy of the DNA sequence. Finally, the transcription must be terminated at the appropriate spots in the genome.
“Termination of transcription is especially important in bacteria because the genes are packed tightly together along the genome, such that failure to terminate transcription at the right locations could lead to inappropriate gene expression,” said Paul Babitzke, professor of biochemistry and molecular biology at Penn State and the leader of the research team.
The mechanisms for termination in bacteria traditionally have been classified as either “factor-dependent,” which relies on a protein called Rho, or intrinsic termination, which was thought to be “factor-independent.” Intrinsic termination relies on an RNA hairpin structure that forms in the RNA molecule being produced, which causes the RNA to be released from the transcription machinery.
NusG and NusA are both proteins classified as transcription factor that help regulate gene expression and are part of the complex of proteins that read and transcribe DNA during elongation. NusA interacts with the RNA molecule being produced and NusG can bind to the DNA to pause elongation. Based on previous research, NusA is thought to play a role in intrinsic termination by aiding in the formation of the RNA hairpin, but the role of NusG in termination had not been established.
To further explore the role of these two proteins in intrinsic termination, the research team produced strains of bacteria that lacked NusA, lacked NusG, and lacked both NusA and NusG. They then used a technique that they invented called “Term-Seq,” in which they can preserve and identify the ends of all the RNA molecules produced in their bacterial strains. The RNA ends from the mutant strains could then be compared to RNA molecules from bacteria with normally functioning NusA and NusG.
“We found that some intrinsic termination sites were dependent on NusA, some on NusG, some on either NusA or NusG, and some required both,” said Mandell. “We were somewhat surprised by how big of a role these two proteins play in intrinsic termination. A total of 88% of all intrinsic termination sites relied on NusA or NusG in some capacity. Intrinsic termination is clearly not completely ‘factor-independent.'”
The researchers are still investigating the precise role that the Nus proteins play in transcription termination.
“We think that NusA helps directly in the formation of the hairpins required for intrinsic termination and that NusG is pausing elongation at termination sites to give the hairpin additional time to form,” said Babitzke.
###
In addition to Mandell and Babitzke, the research team includes Rishi Vishwakarma at Penn State, Reid T. Oshiro and Daniel B. Kearns at Indiana University, and Alexander V. Yakhnin and Mikhail Kashlev at the National Cancer Institute. The research was funded by the U.S. National Institutes of Health.
Media Contact
Sam Sholtis
[email protected]
Original Source
https:/
Related Journal Article
http://dx.