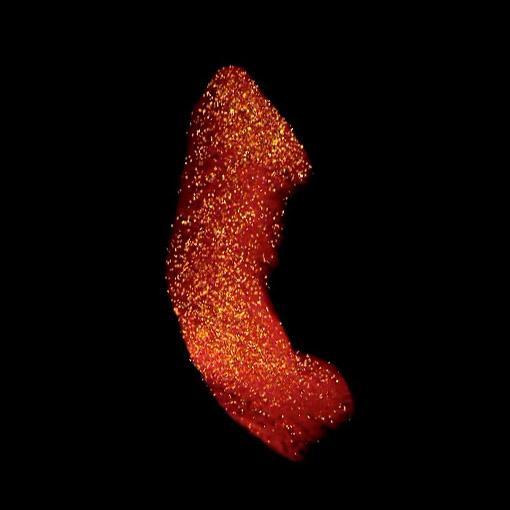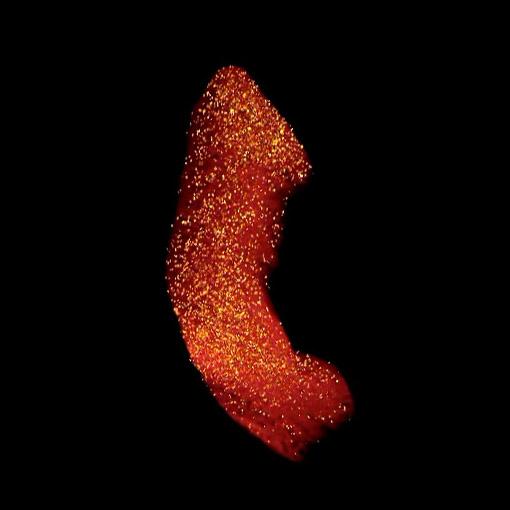
When a blunt-force blow injures the spinal cord, the body's immune system can be both friend and foe. Sensing the injury, the immune system dispatches an inflammatory response composed of specialized cells called macrophages to dispose of dead tissue. However, together with the debris and blood from the initial injury, the macrophages also clear away healthy tissue, resulting in a larger lesion size at the injury site and additional spinal cord injury loss of function.
What if it were possible to reduce the size of the lesion in the spinal cord, thereby preserving more of the spinal cord and nerve function?
Scientists at Case Western Reserve University (CWRU) School of Medicine have demonstrated that a family of therapeutic stem cells called multipotent adult progenitor cells (MAPC®) lessen the consequences of the immune system's damaging second wave response and preserve function that would otherwise be lost. Their findings, which resulted in significant improvements in motor and urinary function in lab animals, appear in the Nov. 19 edition of Scientific Reports, an online journal from the publishers of the journal Nature.
The research team led by Jerry Silver, PhD, professor of neurosciences at CWRU, demonstrated that MAPCs have the ability to modulate the aggressive behavior of macrophages in which they still provided the necessary debris clearing but appear less disruptive to healthy tissue.
"These were kinder, gentler macrophages," Silver said. "They do the job, but they pick and choose what they consume. The end result is spared tissue. We don't know what makes these nicer macrophages more subdued, but this is a subject we are researching in the lab."
Research in the Silver lab, conducted by lead author Marc A. DePaul, also demonstrated that time is a factor in promoting a positive immune response with MAPCs. MAPCs injected into lab animals one day post-injury travelled primarily into their spleens, a reservoir for immature macrophages, resulting in a beneficial macrophage immune response that spared more spinal cord tissue. Consequently, animals that received treatment demonstrated markedly improved hind-paw motor control and urinary function. It takes approximately a day for the immune system to recognize and then begin to respond to a threat caused by injury or illness. When MAPCs were administered too soon (immediately after injury) or not at all (the control group), the lab animals received no benefit.
"There was this remarkable neuroprotection with the friendlier macrophages," Silver said. "The spinal cord was just bigger, healthier, with much less tissue damage."
This most recent research complements a discovery from the Silver lab in 2014 where investigators found that a compound they developed, intracellular sigma peptide (ISP), enhances nerve plasticity and regeneration following spinal cord injury. ISP restored considerable function to lab animals in which the compound was tested.
"Our dream for the future is to combine the neuroprotection of MAPCs with the neurogenerative capacity of ISP," Dr. Silver said. "Both can be delivered systemically, so there is no need to touch the spinal cord. It is already damaged enough."
###
Joining Silver and lead author DePaul in this study were Marc Palmer, Bradley T. Lang, Rochelle Cutrone, Amanda P. Tran, Kathryn M. Madalena, Annelies Bogaerts, Jason A. Hamilton, Robert J. Deans, Robert W. Mays, and Sarah A. Busch. Contributing authors Busch, Deans and Mays hold shares of stock in Athersys, Inc., and Busch, Lang, Palmer, Bogaerts, Cutrone, Deans, and Mays are employees of the company.
This work was supported by an Ohio Third Frontier Grant to the National Center for Regenerative Medicine, the National Institute of Neurological Disorders and Stroke grant NS025713; the Case Western Reserve University Council to Advance Human Health; P. Jing, R. Senior and S. Poon; Unite to Fight Paralysis; The Brumagin Memorial Fund; Spinal Cord Injury Sucks; United Paralysis Foundation; and The Kaneko Family Fund.
The MAPCs were supplied by Athersys Inc., a biotechnology company in Cleveland, Ohio. The Food and Drug Administration has authorized the use of the clinical grade MAPC product, MultiStem®, for clinical trials treating conditions where the immune response is particularly harmful, such as stroke, heart attack and organ transplantation.