When a stroke strikes, every second counts. Strokes remain one of the leading causes of death and disability across the globe, with ischemic strokes making up approximately 90% of all cases. These events occur due to an obstruction impeding blood flow to the brain, triggering a cascade of damage to neuronal tissue. The current frontline treatment involves administering clot-busting drugs, thrombolytics, that must be delivered within a narrow time frame of roughly four and a half hours following the onset of symptoms, often limiting therapeutic opportunities for many patients. Extending this critical window or developing novel strategies to rehabilitate brain tissue after this period remains a paramount challenge in neurology.
In a groundbreaking advance, an international team of researchers has explored a promising avenue using stem cell-based therapy to promote brain repair well beyond this acute period. Conducted in murine models, their study detailed in Nature Communications elucidates how transplantation of human-induced pluripotent stem cell (iPSC)-derived neural progenitors can significantly enhance functional recovery when delivered as late as one week post-ischemic stroke. This delay far exceeds the current therapeutic window for standard interventions and opens new prospects for patients who are ineligible for early treatment or suffer from persistent deficits.
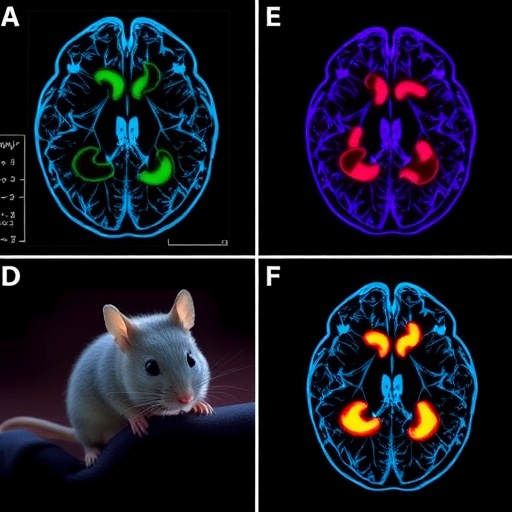
The investigators, including scientists from the Keck School of Medicine of USC, the University of Zurich, and ETH Zurich, employed advanced cellular reprogramming techniques to transform adult human blood cells into neural stem cells capable of differentiating into neurons. These cells were then transplanted directly into the damaged brain regions of stroked mice. Over the course of five weeks post-transplantation, the recipient animals exhibited notable improvements in both neuroanatomical repair and motor function compared to control groups receiving sham surgeries without cell implantation.
At the cellular level, the transplanted neural stem cells facilitated a markedly reduced inflammatory response—a critical factor since unchecked inflammation can exacerbate secondary brain injury after stroke. Their presence encouraged neurogenesis, the generation of new neurons, alongside enhanced angiogenesis, the formation of new blood vessels, both crucial for reconstructing the injured neurovascular environment. Furthermore, treated mice demonstrated improved synaptic connectivity, indicating the re-establishment of functional neural circuits, which underpin recovery of neurological capabilities.
One of the notable physiological benefits observed was the reduction of blood-brain barrier (BBB) permeability in treated animals. The BBB is a selective interface protecting the brain from harmful substances circulating in the bloodstream, and its disruption following ischemic injury contributes to edema and infiltration of neurotoxic agents. By preserving BBB integrity, the stem cell transplantation exerted a protective effect that is essential for sustained brain function and repair.
To rigorously assess functional recovery, the research team applied state-of-the-art artificial intelligence-driven behavioral analyses. These deep learning tools have revolutionized the precision of tracking subtle motor impairments and improvements in preclinical models. Specifically, the treated mice regained fine motor skills necessary for navigating a challenging ladder with irregular rungs, a task requiring coordinated limb control and balance. Additionally, improvements in gait patterns were evident, further signaling a return of complex motor coordination after stroke-induced impairment.
Underlying these functional recoveries are intriguing findings regarding the fate and dynamics of the transplanted stem cells themselves. The stroke injury primarily destroys a subset of inhibitory interneurons known as GABAergic neurons, which regulate excitatory neuronal activity and contribute to overall network stability and plasticity during recovery phases. Remarkably, a significant portion of iPSC-derived cells matured into these GABAergic neurons, potentially guided by local environmental cues within the injured cerebral tissue. This selective differentiation might be critical in restoring the excitation-inhibition balance disrupted by stroke.
Beyond cellular fate, molecular analyses uncovered heightened activity in several intrinsic signaling pathways associated with neuronal regeneration, synaptogenesis, and dendritic arborization. These molecular cascades have long been implicated in neuroplasticity and rewiring of brain circuits after injury. The team’s mechanistic insights suggest that the engrafted cells do not merely replace lost neurons but actively interact with the host brain milieu to stimulate endogenous repair processes.
Such mechanistic understanding is pivotal for advancing regenerative therapies. It paves the way for combinatory approaches where pharmacological agents, potentially repurposed from other diseases, could be employed to amplify the beneficial signaling networks initiated by stem cell grafts. Unraveling these pathways sets the stage for precision medicine strategies designed to optimize treatment efficacy and durability.
The researchers are now embarking on long-term studies to investigate the persistence and integration of transplanted cells over the entire lifespan of the mouse, along with monitoring whether the functional gains are maintained or possibly enhanced with time. These longitudinal studies are critical for translating these findings into human clinical trials, where the safety, stability, and long-term effects of stem cell transplants will be scrutinized.
Dr. Ruslan Rust, a leading figure in this research, emphasizes the translational significance of these findings. “Our goal is to develop a therapy that could be administered to stroke patients well beyond the acute phase, helping those with chronic symptoms or large infarcts to regain meaningful function,” he said. This represents a paradigm shift in stroke treatment, from purely immediate interventions to regenerative therapies targeting long-term recovery.
This research was made possible through collaborative efforts supported by multiple prestigious institutions, including the Swiss 3R Competence Center, the Swiss National Science Foundation, and the Neuroscience Center Zurich. The multidisciplinary team comprised experts in stem cell biology, neurology, bioinformatics, and molecular neuroscience, highlighting the integrated approach necessary to tackle complex neurological disorders.
As stroke continues to impart devastating consequences worldwide, innovations such as these cutting-edge stem cell therapies shine a hopeful light on future clinical possibilities. By bridging fundamental science with clinical aspirations, this study lays critical groundwork for new regenerative treatments capable of restoring brain function well after the initial injury window has closed, ultimately improving quality of life for millions of stroke survivors.
Subject of Research: Animals
Article Title: Human iPSC-derived cell grafts promote functional recovery by molecular interaction with stroke-injured brain
Web References:
10.1038/S41467-025-63725-3
Keywords: Cerebrovascular disorders, Brain, Blood brain barrier, Brain ischemia, Stem cells, Neurons, GABAergic neurons
Tags: advanced stroke interventionsdelayed treatment for stroke recoveryfunctional recovery in stroke patientshuman-induced pluripotent stem cellsischemic stroke recovery strategiesmurine models in stroke researchneurological rehabilitation after strokeneuronal tissue regenerationnovel approaches to brain repairstem cell therapy for brain regenerationstroke-related disability solutionsthrombolytics and stroke treatment