In order to convert sunlight into electricity or other forms of energy as efficiently as possible, the very first step is an efficient light-harvesting system. Ideally, this should be panchromatic, i.e. absorb the entire spectrum of visible light.
Credit: Alexander Schulz / University of Wuerzburg
In order to convert sunlight into electricity or other forms of energy as efficiently as possible, the very first step is an efficient light-harvesting system. Ideally, this should be panchromatic, i.e. absorb the entire spectrum of visible light.
The light-collecting antennae of plants and bacteria are a model for this. They capture a broad spectrum of light for photosynthesis, but are very complex in structure and require many different dyes to transmit the energy of the absorbed light and focus it on a central point.
The light-harvesting systems developed by humans to date also have disadvantages:
Although inorganic semiconductors such as silicon are panchromatic, they only absorb light weakly. In order to absorb enough light energy, very thick layers of silicon in the micrometre range are therefore required – making solar cells relatively bulky and heavy.
Organic dyes that are suitable for solar cells are much thinner: their layer thickness is only around 100 nanometres. However, they are barely able to absorb a broad spectral range and are therefore not particularly efficient.
Thin Layer Absorbs a Lot of Light Energy
Researchers at Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany, in the journal Chem have now presented an innovative light-harvesting system that differs significantly from previous systems.
“Our system has a band structure similar to that of inorganic semiconductors. This means that it absorbs light panchromatically across the entire visible range. And it uses the high absorption coefficients of organic dyes. As a result, it can absorb a great deal of light energy in a relatively thin layer, similar to natural light-harvesting systems,” says JMU chemistry professor Frank Würthner. His team from the Institute of Organic Chemistry / Center for Nanosystems Chemistry designed the light-harvesting system at JMU and investigated it together with Professor Tobias Brixner’s group from the Institute of Physical and Theoretical Chemistry.
Four Dyes in an Ingenious Arrangement
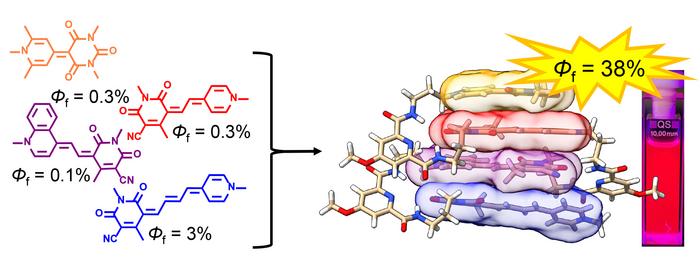
Put simply, the innovative light-harvesting antenna from Würzburg consists of four different merocyanine dyes that are folded and thereby stacked closely together. The elaborate arrangement of the molecules enables ultra-fast and efficient energy transport within the antenna.
The researchers have given the prototype of the new light-harvesting system the name URPB. The letters stand for the light wavelengths that are absorbed by the four dye components of the antenna: U for ultraviolet, R for red, P for purple, B for blue.
Proven Performance Via Fluorescence
The researchers have demonstrated that their novel light-collecting system works so well by measuring the so-called fluorescence quantum yield. This involves measuring how much energy the system emits in the form of fluorescence. This allows conclusions to be drawn about the amount of light energy that it has previously collected.
The result: the system converts 38 per cent of the irradiated light energy over a broad spectral range into fluorescence – the four dyes on their own, on the other hand, manage less than one per cent to a maximum of three per cent. The right combination and skilful spatial arrangement of dye molecules in the stack therefore make a big difference.
Journal
Chem
DOI
10.1016/j.chempr.2024.05.023
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Panchromatic Light-Harvesting Antenna by Supramolecular Exciton Band Engineering for Heteromeric Dye Foldamer
Article Publication Date
26-Jun-2024



