Beta cells in the pancreas are responsible for producing the vital hormone insulin. In diabetes, these cells are either destroyed or functionally impaired, resulting in elevated blood sugar levels in the body. Researchers led by Principal Investigator Stefan Kubicek at CeMM, the Research Center for Molecular Medicine of the Austrian Academy of Sciences, have now shown that alpha cells, which are also located in the pancreas, can be stimulated to produce insulin by targeting the chromatin protein SMNDC1. The study, published in Cell Reports, identifies a new molecular mechanism regulating the insulin hormone that plays an essential role for the treatment of diabetes
Credit: Tamara Casteels, published in Cell Report 2022.
Beta cells in the pancreas are responsible for producing the vital hormone insulin. In diabetes, these cells are either destroyed or functionally impaired, resulting in elevated blood sugar levels in the body. Researchers led by Principal Investigator Stefan Kubicek at CeMM, the Research Center for Molecular Medicine of the Austrian Academy of Sciences, have now shown that alpha cells, which are also located in the pancreas, can be stimulated to produce insulin by targeting the chromatin protein SMNDC1. The study, published in Cell Reports, identifies a new molecular mechanism regulating the insulin hormone that plays an essential role for the treatment of diabetes
Insulin, a hormone of the pancreas, regulates blood glucose levels in the body and influences numerous metabolic processes both directly and indirectly. Beta cells are the cell type in the pancreas responsible for insulin production and secretion. In type 1 diabetes, these beta cells are destroyed by an autoimmune reaction, but also in type 2 diabetes their function is impaired. Regenerating functional beta cell mass has therefore become a main aim of diabetes research.
Beta cells are found in the pancreas, specifically within cell clusters known as islets of Langerhans. These islets also contain other hormone-producing cells – or endocrine cells – that are closely related to beta cells. One of these cell types is alpha cells, which produce glucagon, a hormone that functionally counteracts insulin. Previous research has shown that alpha cells can be converted into beta cells by genetic overexpression involving a combination of transcription factors. However, this approach cannot easily be translated into therapeutic options.
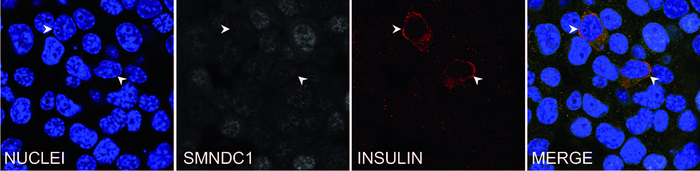
Tamara Casteels, PhD student in the Kubicek lab, investigated the fundamental question of which factors repress insulin production in alpha cells, to identify factors that when inhibited could induce the expression of this important hormone. She focused on chromatin binders and modifiers, that localize directly to DNA, and thereby potentially also bind the insulin gene. Compared to transcription factors, the structures of these chromatin proteins make them theoretically easier to target therapeutically.
Loss of ATRX leads to induction of insulin
Tamara Casteels was able to show that reducing the expression of the chromatin protein SMNDC1 leads to the insulin gene being switched on in alpha cells. This effect was observed not only in a mouse cell line, but also in primary human islets of Langerhans. SMNDC1 is a known splicing factor that has not been extensively studied previously. Stefan Kubicek explains:“Splicing is a step in mRNA processing in which the non-coding intron sequences are cut out of the pre-mRNA. We have shown that the knockdown of SMNDC1 leads to splicing changes at hundreds of genes. Interestingly, one of these genes encodes the chromatin remodeler ATRX, to which SMNDC1 also binds directly. We find that loss of SMNDC1 reduces the abundance of both ATRX mRNA and ATRX protein. This, in turn, causes upregulation of the important beta cell transcription factor PDX1, which is well-known to stimulate insulin production.”
Further effects of the knock-down of SMNDC1 still open
This publication brings important fundamental insights into the regulation of insulin in alpha cells. Initial attempts to regulate SMNDC1 in beta cells further indicate that the protein also influences insulin production there. However, before considering translational opportunities regarding potential future therapeutic use of an SMNDC1 knockdown for insulin production, many challenges remain. “The amount of insulin induced in alpha cells after SMNDC1 had been knocked-down, are significantly lower than those induced in beta cells”, Kubicek said. “And as an essential gene, complete loss of SMNDC1 can impair the viability of most cell types.”
To better test the dose dependence effects, the role of SMNDC1 in other tissues, and the potential for combination with other factors that influence alpha cell identity, researchers in the Kubicek lab are currently working on the development of small molecule inhibitors of SMNDC1.
__________________________________________________________________
Stefan Kubicek joined CeMM in August 2010. He obtained an MSc in synthetic organic chemistry from the Vienna University of Technology after writing a diploma thesis at ETH Zurich. For his PhD in Thomas Jenuwein’s lab at the IMP in Vienna, he changed fields to molecular biology. He then performed postdoctoral research working on chemical biology with Stuart Schreiber at the Broad Institute of Harvard and MIT. Stefan Kubicek heads the chemical screening platform and PLACEBO (Platform Austria for Chemical Biology), a task he is well-equipped for based on previous screening experience with Boehringer Ingelheim and at the Broad Institute. Stefan Kubicek has also headed the Christian Doppler Laboratory for Chemical Epigenetics and Antiinfectives, a public-private partnership between CeMM, Boehringer Ingelheim and Haplogen. The Kubicek lab is working on the role of chromatin in the definition of cell types and cell states, particularly chromatin modifying enzymes as synthetic lethal targets in cancer and chemical transdifferentiation to insulin-producing beta cells. In an ERC-funded project, the laboratory is working on metabolic enzymes in the cell’s nucleus and testing the hypothesis that small molecule metabolites shape chromatin structure and thus control gene expression and cell identity.
The CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences is an international, independent and interdisciplinary research institution for molecular medicine under the scientific direction of Giulio Superti-Furga. CeMM is oriented towards medical needs and integrates basic research and clinical expertise to develop innovative diagnostic and therapeutic approaches for precision medicine. Research focuses on cancer, inflammation, metabolic and immune disorders, and rare diseases. The Institute’s research building is located on the campus of the Medical University and the Vienna General Hospital. cemm.at
Journal
Cell Reports
DOI
10.1016/j.celrep.2022.111288
Subject of Research
Cells
Article Title
SMNDC1 links chromatin remodeling and splicing to regulate pancreatic hormone expression
Article Publication Date
30-Aug-2022




