Individual cells divide through a process called mitosis, during which the cell’s copied DNA is separated between two resulting daughter cells. Despite recent advances in cell biology, the mechanism by which DNA condenses during mitosis is still poorly understood. Researchers recently tracked small stretches of DNA wound around histone proteins, called nucleosomes, to better characterize nucleosome behavior during cell division.
Individual cells divide through a process called mitosis, during which the cell’s copied DNA is separated between two resulting daughter cells. Despite recent advances in cell biology, the mechanism by which DNA condenses during mitosis is still poorly understood. Researchers recently tracked small stretches of DNA wound around histone proteins, called nucleosomes, to better characterize nucleosome behavior during cell division.
DNA is organized as chromatin, which are dynamic structures comprised of DNA, RNA, and proteins that regulate the accessibility of genes for expression and the overall configuration of genetic material in the cell. Histone proteins, for example, are positively charged proteins that bind to negatively charged DNA. DNA wraps around these histone proteins to form nucleosomes, which help condense nearly six feet of human genomic DNA into a nucleus only 10 micrometers (1 x 10-6 m) across.
During mitosis, DNA condenses before being divided between two daughter cells. A protein complex called condensins is involved in assembling the condensed chromosomes. However, researchers are still unsure how cells achieve chromosome assembly during cell division. To address this, a team of researchers from the National Institute of Genetics in Mishima, Japan, part of the Research Organization of Information and Systems (ROIS), used single-nucleosome imaging to reveal the factors that contribute to the organization and compaction of chromosomes during mitosis in living cells (Movie).
The team published the study on August 25 in Nature Communications.
DOI: https://doi.org/10.1038/s41467-024-51454-y
“Mitotic chromosome assembly is an essential process for transmitting replicated chromosomes to two daughter cells during cell division. While protein factors like condensins play key roles in this process, it is unclear how nucleosomes, the building blocks of chromatin, behave during chromosome assembly and how condensins act on nucleosomes to organize chromosomes. To study these points, we tracked the movement of individual nucleosomes during cell division in living human cells using super-resolution microscope,” said Kazuhiro Maeshima, professor at the National Institute of Genetics and SOKENDAI (the Graduate University for Advanced Studies) in Mishima, Japan.
The team observed that nucleosomes are much more constrained during mitosis compared to cells in interphase. Nucleosomes were most constrained when chromosomes were being moved to opposite poles of the cell during anaphase, a specific phase of cell division. These constraints were loosened during telophase, the last phase of cell division, when chromosome decompaction begins.
The team also performed condensin-depletion experiments to investigate the constraining process during mitosis. They found that depleting condensins caused abnormal chromosome shapes and increased nucleosome motion. This observation supports a model of chromosome organization in which condensins form loops to constrain nucleosomes. Importantly, Yuji Sakai from the Yokohama City University was able to recapitulate their observations using computational modeling (Movie).
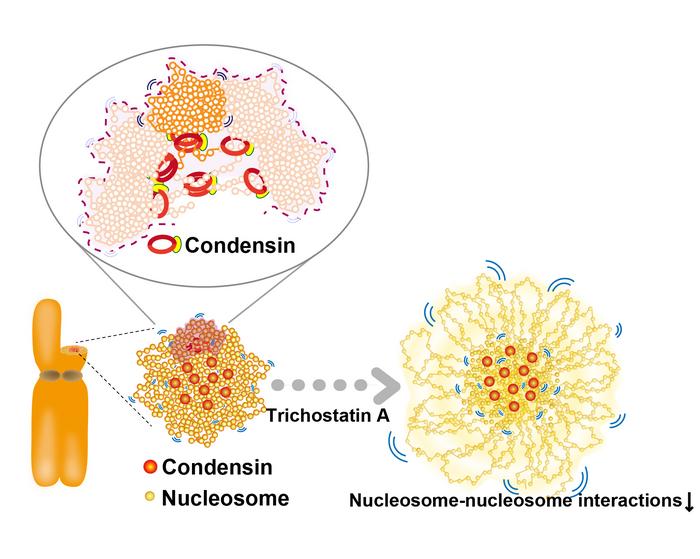
“Our findings revealed that as chromosomes are assembled during cell division, nucleosome movement becomes increasingly restricted. Condensins function like ‘molecular crosslinkers,’ holding nucleosomes in place to organize the chromosomes. Additionally, interactions between nucleosomes, facilitated by the tails of histone proteins, help further compact the chromosomes (Figure),” said Kayo Hibino from the National Institute of Genetics and SOKENDAI.
The team’s computational modeling and the lack of condensins at the periphery of chromosomes suggest that other constraining factors may contribute to chromosome condensation during mitosis. By reducing the positive charge on histone proteins with the reagent trichostatin A (TSA), researchers observed increased nucleosome movement (Figure), similar to the results of condensin depletion experiments.
Additional contributors to this research include Katsuhiko Minami, Masa A. Shimazoe from the Genome Dynamics Laboratory at the National Institute of Genetics and the Graduate Institute for Advanced Studies at SOKENDAI, both in Mishima, Japan; Sachiko Tamura from the Genome Dynamics Laboratory at the National Institute of Genetics; Masatoshi Takagi from the Cellular Dynamics Laboratory at the RIKEN Cluster for Pioneering Research in Wako, Japan and the Laboratory for Cell Function Dynamics at the RIKEN Center for Brain Science in Wako, Japan; Toyoaki Natsume from the Graduate Institute for Advanced Studies at SOKENDAI, the Molecular Cell Engineering Laboratory at the National Institute of Genetics in Mishima, Japan and the Research Center for Genome & Medical Sciences at the Tokyo Metropolitan Institute of Medical Science in Tokyo, Japan; Masato T. Kanemaki from the Graduate Institute for Advanced Studies at SOKENDAI, the Molecular Cell Engineering Laboratory at the National Institute of Genetics in Mishima, Japan and the Department of Biological Science at The University of Tokyo in Tokyo, Japan; and Naoko Imamoto from the Cellular Dynamics Laboratory at the RIKEN Cluster for Pioneering Research and the Graduate School of Medical Safety Management at Jikei University of Health Care Sciences in Osaka, Japan.
###
About National Institute of Genetics (NIG)
National Institute of Genetics (NIG) was established to carry out broad and comprehensive research in genetics. NIG contributes to the development of academic research as one of the inter-university research institutes constituting the Research Organization of Information and Systems (ROIS).
About the Research Organization of Information and Systems (ROIS)
ROIS is a parent organization of four national institutes (National Institute of Polar Research, National Institute of Informatics, the Institute of Statistical Mathematics and National Institute of Genetics) and the Joint Support-Center for Data Science Research. It is ROIS’s mission to promote integrated, cutting-edge research that goes beyond the barriers of these institutions, in addition to facilitating their research activities, as members of inter-university research institutes.
Journal
Nature Communications
DOI
10.1038/s41467-024-51454-y