WASHINGTON, Nov. 1, 2022 – Many medical devices for treating heart failure generate nonphysiological shear flow. This can trigger the destruction of red blood cells after implantation of ventricular assist devices (VADs), artificial heart valves, vascular stents, or interventional thrombectomy devices.
Credit: Zhike Xu, Chenyang Wang, Sen Xue, Feng He, Pengfei Hao, and Xiwen Zhang
WASHINGTON, Nov. 1, 2022 – Many medical devices for treating heart failure generate nonphysiological shear flow. This can trigger the destruction of red blood cells after implantation of ventricular assist devices (VADs), artificial heart valves, vascular stents, or interventional thrombectomy devices.
The destruction of red blood cells, or mechanical hemolysis, is an inevitable complication of interventional devices, so scientists want to gain a better understanding of the phenomenon.
In Physics of Fluids, from AIP Publishing, researchers from Tsinghua University developed a red blood cell destruction model based on simulations of dissipative particle dynamics within a high shear flow. They used the results to make recommendations for improvements of VADs.
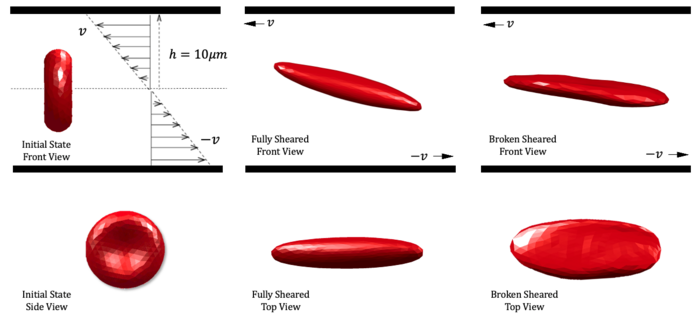
“After interventional medical devices are implanted inside the human body, the nearby flow field generates a shear flow with a very high shear rate,” said co-author Xiwen Zhang. “The velocity change rate of the fluid will deform the red blood cell membrane. Eventually, deformation of the membrane exceeds the ultimate strain, and the membrane is disrupted by shear flow.”
The team discovered that acceleration during shearing is a major factor in red blood cell destruction, beyond exposure time and shear stress. They recommend adding a flow buffer structure to the structural design of VADs to reduce part of the hemolysis caused by shear acceleration.
For hemolysis-related research, many researchers focus on macroscale experiments to obtain a series of empirical fitting formulas.
“But our team is exploring the shear destruction process of red blood cells in more detail at the red blood cell scale by using dissipative particle dynamics,” said Zhang.
“We hope our study can serve as a bridge between macroscopic hemolysis experiments and microscopic red blood cell simulations (molecular dynamics simulations),” said Zhang. “In future work, we will continue constructing shear failure models of multiple red blood cells and perform shear failure simulations based on whole blood to be able to compare them with macroscopic hemolysis experiments.”
The researchers are currently developing a new index to predict the hemolysis of VADs more accurately and help optimize the shape of VADs, which should improve hydraulic performance and reduce hemolysis.
They plan to better represent the diffusion process of hemoglobin after shear damage by adding a transport dissipation particle dynamics model based on this work.
###
The article “The erythrocyte destruction mechanism in non-physiological shear mechanical hemolysis” is authored by Zhike Xu, Chenyang Wang, Sen Xue, Feng He, Pengfei Hao, and Xiwen Zhang. The article will appear in Physics of Fluids on Nov. 1, 2022 (DOI: 10.1063/5.0112967). After that date, it can be accessed at https://aip.scitation.org/doi/10.1063/5.0112967.
ABOUT THE JOURNAL
Physics of Fluids is devoted to the publication of original theoretical, computational, and experimental contributions to the dynamics of gases, liquids, and complex fluids. See https://aip.scitation.org/journal/phf.
###
Journal
Physics of Fluids
DOI
10.1063/5.0112967
Article Title
The erythrocyte destruction mechanism in non-physiological shear mechanical hemolysis
Article Publication Date
1-Nov-2022




