The remarkable proton and oxide-ion (dual-ion) conductivities of hexagonal perovskite-related oxide Ba7Nb3.8Mo1.2O20.1 are promising for next-generation electrochemical devices, as reported by scientists at Tokyo Tech. The unique ion-transport mechanisms they unveiled will hopefully pave the way for better dual-ion conductors, which could play an essential role in tomorrow’s clean energy technologies.
Credit: Chemistry of Materials
The remarkable proton and oxide-ion (dual-ion) conductivities of hexagonal perovskite-related oxide Ba7Nb3.8Mo1.2O20.1 are promising for next-generation electrochemical devices, as reported by scientists at Tokyo Tech. The unique ion-transport mechanisms they unveiled will hopefully pave the way for better dual-ion conductors, which could play an essential role in tomorrow’s clean energy technologies.
Clean energy technologies are the cornerstone of sustainable societies, and solid-oxide fuel cells (SOFCs) and proton ceramic fuel cells (PCFCs) are among the most promising types of electrochemical devices for green power generation. These devices, however, still face challenges that hinder their development and adoption.
Ideally, SOFCs should be operated at low temperatures to prevent unwanted chemical reactions from degrading their constituent materials. Unfortunately, most known oxide-ion conductors, a key component of SOFCs, only exhibit decent ionic conductivity at elevated temperatures. As for PCFCs, not only are they chemically unstable under carbon dioxide atmospheres, but they also require energy-intensive, high-temperature processing steps during manufacture.
Fortunately, there is a type of material that can solve these problems by combining the benefits of both SOFCs and PCFCs: dual-ion conductors. By supporting the diffusion of both protons and oxide ions, dual-ion conductors can realize high total conductivity at lower temperatures and improve the performance of electrochemical devices. Although some perovskite-related dual-ion conducting materials such as Ba7Nb4MoO20 have been reported, their conductivities are not high enough for practical applications, and their underlying conducting mechanisms are not well understood.
Against this backdrop, a research team led by Professor Masatomo Yashima from Tokyo Institute of Technology, Japan, decided to investigate the conductivity of materials similar to 7Nb4MoO20 but with a higher Mo fraction (that is, Ba7Nb4-xMo1+xO20+x/2). Their latest study, which was conducted in collaboration with the Australian Nuclear Science and Technology Organisation (ANSTO), the High Energy Accelerator Research Organization (KEK), and Tohoku University, was published in Chemistry of Materials.
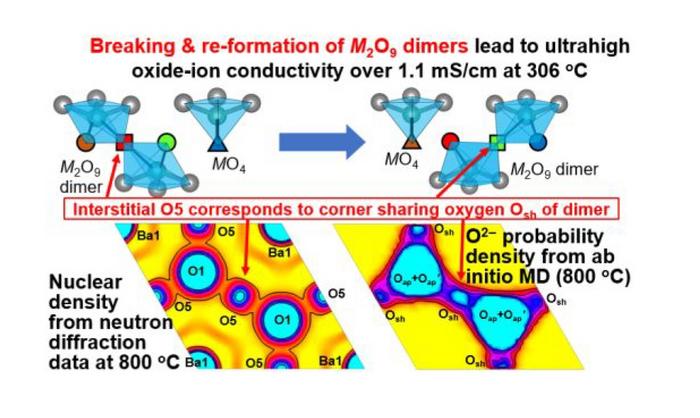
After screening various Ba7Nb4-xMo1+xO20+x/2 compositions, the team found that Ba7Nb3.8Mo1.2O20.1 had remarkable proton and oxide-ion conductivities. “Ba7Nb3.8Mo1.2O20.1 exhibited bulk conductivities of 11 mS/cm at 537 ℃ under wet air and 10 mS/cm at 593 ℃ under dry air. Total direct current conductivity at 400 ℃ in wet air of Ba7Nb3.8Mo1.2O20.1 was 13 times higher than that of Ba7Nb4MoO20, and the bulk conductivity in dry air at 306 ℃ is 175 times higher than that of the conventional yttria-stabilized zirconia (YSZ),” highlights Prof. Yashima.
Next, the researchers sought to shed light on the underlying mechanisms behind these high conductivity values. To this end, they conducted ab initio molecular dynamics (AIMD) simulations, neutron diffraction experiments, and neutron scattering length density analyses. These techniques enabled them to study the structure of Ba7Nb3.8Mo1.2O20.1 in greater detail and determine what makes it special as a dual-ion conductor.
Interestingly, the team found that the high oxide-ion conductivity of Ba7Nb3.8Mo1.2O20.1 originates from a unique phenomenon (Figure). It turns out that adjacent MO5 monomers in Ba7Nb3.8Mo1.2O20.1 can form M2O9 dimers by sharing an oxygen atom on one of their corners (M = Nb or Mo cation). The breaking and reforming of these dimers gives rise to ultrafast oxide-ion movement in a manner analogous to a long line of people relaying buckets of water (oxide ions) from one person to the next. Furthermore, the AIMD simulations revealed that the observed high proton conduction was due to efficient proton migration in the hexagonal close-packed BaO3 layers in the material.
Taken together, the results of this study highlight the potential of perovskite-related dual-ion conductors and could serve as guidelines for the rational design of these materials. “The present findings of high conductivities and unique ion migration mechanisms in Ba7Nb3.8Mo1.2O20.1 will help the development of science and engineering of oxide-ion, proton, and dual-ion conductors,” concludes a hopeful Prof. Yashima.
We hope further research leads us to even better conducting materials for next-generation energy technologies.
###
Yashima Research Group
About Tokyo Institute of Technology
Tokyo Tech stands at the forefront of research and higher education as the leading university
for science and technology in Japan. Tokyo Tech researchers excel in fields ranging from
materials science to biology, computer science, and physics. Founded in 1881, Tokyo Tech
hosts over 10,000 undergraduate and graduate students per year, who develop into scientific
leaders and some of the most sought-after engineers in industry. Embodying the Japanese
philosophy of “monotsukuri,” meaning “technical ingenuity and innovation,” the Tokyo Tech
community strives to contribute to society through high-impact research.
https://www.titech.ac.jp/english/
Journal
Chemistry of Materials
DOI
10.1021/acs.chemmater.3c02378
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Dimer-Mediated Cooperative Mechanism of Ultrafast-Ion Conduction in Hexagonal Perovskite-Related Oxides
Article Publication Date
14-Nov-2023




