LA JOLLA, CA—Chemists from Scripps Research and the University of California, Los Angeles, have developed methods for the precise, flexible modification of a broad class of chemical compounds called bicyclic aza-arenes, which are commonly used to build drug molecules.
Credit: Scripps Research
LA JOLLA, CA—Chemists from Scripps Research and the University of California, Los Angeles, have developed methods for the precise, flexible modification of a broad class of chemical compounds called bicyclic aza-arenes, which are commonly used to build drug molecules.
The landmark achievement, reported August 9, 2022, in Nature, reflects a powerful new approach that generally offers much easier and more flexible molecular design, enabling chemists to synthesize innumerable chemical products—including potential blockbuster drugs—that were previously out of reach.
“These new methods effectively give chemists a unified, practical, late-stage ‘molecular editing’ toolkit for modifying bicyclic aza-arenes at desired sites in any desired order—greatly expanding the diversity of drugs and other useful molecules that could be built from these popular starting compounds,” says study co-leader Jin-Quan Yu, PhD, the Bristol Myers Squibb Endowed Chair in Chemistry and Frank and Bertha Hupp Professor of Chemistry at Scripps Research.
Yu and his lab collaborated on the research with the lab of Kendall Houk, PhD, Distinguished Research Professor in the Department of Chemistry and Biochemistry at UCLA. The first authors of the study were postdoctoral researchers Zhoulong Fan, PhD, and Xiangyang Chen, PhD, of the Yu and Houk labs respectively.
Building organic molecules with laboratory chemistry techniques, a practice known as organic synthesis, has always been more challenging than building things at macro scale. Down at the molecular scale, how sets of atoms move and bond to each other is governed by a highly complex mix of forces. Although chemists have developed hundreds of reactions that can transform starting compounds into other compounds, they have lacked toolkits for modifying widespread carbon centers containing carbon-hydrogen bonds only.
The ambitious goal, or “Holy Grail,” of many synthetic chemists has been to develop flexible and universal molecular editing methods that modify as many carbon atoms as possible at any site by breaking carbon-hydrogen bonds in the starting molecules. Specifically, synthetic chemists have wanted to, in a streamlined and easy way, modify the atom of their choice—typically carbon—on the backbone of a given organic molecule, and to modify more than one of these carbon atoms on the molecule, and in any order. This ability would make the construction of new molecules as straightforward as creating a sentence by changing individual words at will. But the difficulty of devising reactions that can direct a modification to one specific atom, and not others that may be virtually identical in traditional chemical terms, has tended to make the concept of molecular editing seem like an impossible dream.
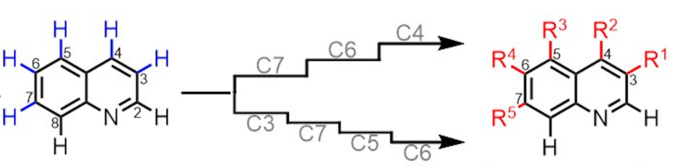
The new method has turned this dream into reality, at least with respect to one of the most common classes of starting molecule used by pharmaceutical chemists. Bicyclic aza-arenes are relatively simple organic molecules that include two ring-like backbones, mostly made of carbon atoms but with at least one nitrogen atom. Myriad existing drugs and medically relevant natural compounds are built from bicyclic aza-arene scaffolds.
The new methods allow chemists to selectively modify multiple carbon atoms, when they are bound to simple hydrogen atoms, at various sites on bicyclic aza-arenes. The flexible modification of these sites permits novel, potentially pharmaceutically relevant structures that were previously difficult to synthesize.
The new methods are variants of an approach called CH (carbon-hydrogen) functionalization: removing a standard hydrogen atom from a carbon atom and replacing it with a more complex set of atoms. CH functionalization is conceptually the most straightforward way of adding complexity to a starting molecule, and the Yu laboratory is known for its many innovations in this field. The new methods employ specially designed helper molecules called directing templates that become reversibly anchored to the starting molecule, and, like construction cranes, efficiently direct CH functionalization at the desired sites. The templates are considered “catalytic” because they direct the reactions but are not consumed by them, and thus continue to work without the need for constant replenishment.
“A key aspect of our new approach is that the templates direct CH functionalization not based on traditional electronic criteria, but instead on the distance and geometry of the path to the target,” Yu says.
The new set of techniques should be easy for chemists to use, and should be adopted rapidly by the pharma industry and other chemistry-based industries, he adds.
“We also expect soon to broaden this approach to other classes of starting compounds,” Yu says.
“Molecular editing of multiple C–H bonds by distance, geometry and chirality” was co-authored by Zhoulong Fan, Keita Tanaka, Han Seul Park, Nelson Lam, and Jin-Quan Yu, of Scripps Research; and by Xiangyang Chen, Jonathan Wong, and K. N. Houk of UCLA.
Funding for the research was provided by the National Institutes of Health (R01 GM102265) and the National Science Foundation (CHE-1764328, CHE-1700982).
About Scripps Research
Scripps Research is an independent, nonprofit biomedical institute ranked the most influential in the world for its impact on innovation by Nature Index. We are advancing human health through profound discoveries that address pressing medical concerns around the globe. Our drug discovery and development division, Calibr, works hand-in-hand with scientists across disciplines to bring new medicines to patients as quickly and efficiently as possible, while teams at Scripps Research Translational Institute harness genomics, digital medicine and cutting-edge informatics to understand individual health and render more effective healthcare. Scripps Research also trains the next generation of leading scientists at our Skaggs Graduate School, consistently named among the top 10 US programs for chemistry and biological sciences. Learn more at www.scripps.edu.
Journal
Nature
Article Title
Molecular editing of multiple C–H bonds by distance, geometry and chirality
Article Publication Date
9-Aug-2022




