In an exciting breakthrough, chemists at Scripps Research have revealed a novel approach to enhancing the reactivity of ketones and esters, two fundamental classes of molecules that are crucial in pharmaceutical development and organic synthesis. Historically regarded as relatively inert, which limited their utility in chemical reactions, these molecules are now being reassessed in light of significant new findings that promise to accelerate drug development processes and produce more environmentally sustainable chemical production methods.
For years, ketones and esters have been viewed as repositories of potential, essential for the synthesis of various drug compounds. Their chemical structures, however, often pose a challenge. Chemists traditionally had access to only two sites—namely the carbonyl carbon and the alpha position—making it difficult to initiate chemical transformations at other sites. These restrictions were primarily due to the stability of carbon-hydrogen (C-H) bonds that are prevalent in these compounds, which do not engage readily with catalysts—critical agents in speeding up chemical reactions.
The research team at Scripps has made pioneering advances by developing an innovative method that unlocks the less accessible parts of ketones and esters, effectively broadening their utility in synthetic chemistry. The findings, set to be published in the esteemed journal Nature, elucidate how this new method enhances the potential of these compounds without the need for cumbersome extra steps that have commonly been employed in chemical transformations. This marks a significant milestone in the field of organic chemistry and opens new pathways for chemical synthesis.
The senior author of the study, Dr. Jin-Quan Yu, emphasizes the importance of ketones in chemical synthesis, describing them as foundational yet underutilized entities in the synthesis of new molecules. By overcoming the challenges posed by their unreactive sites, this innovative approach holds the potential to revolutionize the way chemists manipulate these essential compounds, contributing significantly to advancements in drug-research methodologies. Dr. Yu’s perspective ties ketones and esters directly to the heart of pharmaceutical innovation.
This new methodology is built on a foundation of past research conducted by Dr. Yu and his team, who are recognized leaders in the field of C-H activation—a process that revolves around modifying stable C-H bonds to allow for the introduction of various functional groups into a molecule. This discipline has informed a range of strategies for transforming traditionally inert chemical groups into versatile tools for synthetic applications. The new work represents a leap forward in this ongoing effort.
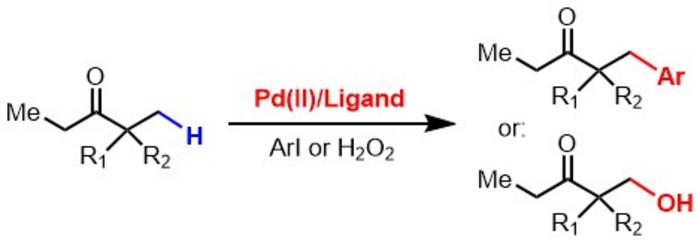
In the chemists’ exploration, a clever use of catalysts has emerged as a centerpiece in their strategy. The specially engineered palladium catalysts used in the new method are designed to bind more effectively to ketones and esters than previously possible, addressing a critical limitation that has hampered attempts to activate these compounds. The researchers additionally mixed in a strong acid known as tetrafluoroboric acid, which stabilizes the catalyst momentarily, allowing for the necessary chemical modifications to take place.
To showcase the power of this innovative system, the research team demonstrated its capacity to induce various chemical reactions like arylation and hydroxylation. These reactions are essential for adding functional groups that can enhance the utility of drug compounds and introduce new functionality into the molecular structures. The implications for drug production are immense, suggesting a shift towards simpler, more efficient synthetic routes that can aid in the customization of chemical compounds to better meet biological requirements.
By enabling direct modifications of ketones and esters in a streamlined manner, this research contributes to the growing body of work aimed at reducing chemical waste—a pressing concern in modern synthetic chemistry that strives for both economic and environmental sustainability. This breakthrough dovetails with broader initiatives within the scientific community to embrace greener methods of chemical production and minimize the ecological footprint of the pharmaceutical industry.
Beyond its direct implications for medicinal chemistry, the study’s findings could extend to a diverse array of fields, including materials science and agrochemicals, as well as the production of common household items. This versatility reinforces the notion that the impact of such research transcends traditional boundaries, opening avenues that could redefine how we understand and utilize chemical compounds in our daily lives.
The overarching goal of Dr. Yu and his lab is not only to optimize current methods but also to explore the creation of chiral molecules—structures that are mirror images of one another, yet have different properties. This endeavor is crucial in the synthesis of many pharmaceuticals, where the orientation of atoms within a molecule can significantly influence its effectiveness as a drug. By adapting their innovative system to facilitate the production of such molecules, the team hopes to unlock an even broader spectrum of synthetic possibilities.
This exciting new methodology for functionalizing ketones and esters promises to not only enhance their applications within synthetic organic chemistry but also signify a paradigm shift in how chemists approach the modification of inert molecules. The research encapsulates a vision of the future where complex molecules can be constructed with greater precision, efficiency, and environmental responsibility. The unfolding of this new chapter in chemical synthesis holds great promise for both the pharmaceutical industry and the broader field of organic chemistry.
As the Scripps Research team prepares to share their findings with the scientific community, the excitement surrounding their work is palpable. The journey of discovering new catalytic methods, unlocking the potential of vulnerable sites on ketones and esters, and paving the way for future advancements is a testament to the innovative spirit of modern chemistry. The evolution of these chemical compounds underscores the importance of research as a catalyst for progress and innovation in the ever-expanding domain of science and technology.
In summary, the research highlights not only the power of ketones and esters but also the collective advancement of knowledge that enables scientists to push the boundaries of what was previously thought possible. As the field continues to develop, there is no telling what other transformative breakthroughs may arise from this profound exploration of chemical synthesis.
Subject of Research: Functionalization of ketones and esters
Article Title: β-C−H bond functionalization of ketones and esters by cationic Pd complexes
News Publication Date: 8-Jan-2025
Web References: Nature
References: Supported by the National Institute of General Medical Sciences (grant 2R01GM084019).
Image Credits: Credit: Scripps Research
Keywords
Ketones, Esters, C-H Activation, Catalysis, Organic Synthesis, Pharmaceutical Chemistry, Green Chemistry, Chiral Molecules, Chemical Modification, Chemical Innovation.