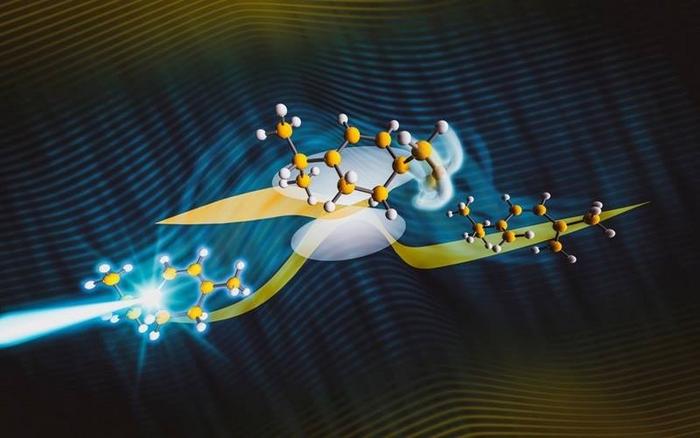
In chemical reactions, molecules proceed during their transformation from reactants into reaction products through a critical geometry. In chemistry, geometry refers to the arrangement of atoms in a molecule. Scientists often call critical geometry in reactions a transition state. This state has an almost incomprehensibly short lifetime of less than one millionth of one millionth of a second. Scientists recently captured a critical geometry using the ultra-high speed “electron camera” at SLAC. In combination with quantum simulations of the reaction, this allowed researchers to identify the critical structure as one end of the molecule bending away from the rest of the molecule.
Credit: Image courtesy of Greg Stewart, SLAC National Accelerator Laboratory
The Science
In chemical reactions, molecules proceed during their transformation from reactants into reaction products through a critical geometry. In chemistry, geometry refers to the arrangement of atoms in a molecule. Scientists often call critical geometry in reactions a transition state. This state has an almost incomprehensibly short lifetime of less than one millionth of one millionth of a second. Scientists recently captured a critical geometry using the ultra-high speed “electron camera” at SLAC. In combination with quantum simulations of the reaction, this allowed researchers to identify the critical structure as one end of the molecule bending away from the rest of the molecule.
The Impact
Chemists use the reaction investigated in this study, a so-called electrocyclic reaction, because it generates very specific reaction products. These products can be predicted by the Woodward-Hoffmann rules. These rules received the Nobel Prize in chemistry in 1981 and are taught to every organic chemist during their undergraduate education. However, the rules do not give a detailed answer why reactions generate only specific reaction products. The new results help to address this open question. Additionally, they open a path for researchers to create new rules for other types of reactions. This can help make organic chemistry a more powerful tool.
Summary
Electrocyclic reactions are characterized by the simultaneous formation and dissociation of multiple chemical bonds through one critical geometry. In the case of alpha-terpinene, the molecule studied in this project, two double bonds and one single bond are transformed into three double bonds. The synchronization of these processes and the single critical configuration ensure their stereospecificity, a characteristic that makes them an important tool for synthetic chemistry. The stereospecificity can be predicted by the well-known Woodward-Hoffmann rules.
The present study investigated a photochemical (i.e., light-triggered) electrocyclic ring-opening reaction with a combination of ultrafast electron diffraction and simulations of the reaction dynamics in alpha-terpinene. The Woodward-Hoffmann rules predict that the stereospecificity of the reaction in alpha-terpinene is ensured by a rotation of the ends of the emerging chain-like reaction product away from each other in the same clockwise or counter-clockwise direction. The new results suggest that the origins of the stereospecificity do not lie in the exact nature of the motion. Instead, the stereospecificity is determined by the fact that the change from two to three double bonds has largely already happened when the molecule assumes the critical geometry. The single bond dissociation, which leads to the opening of the alpha-terpinene ring, happens later, during the transformation of the molecule from the critical geometry to the reaction products.
Funding
This work was supported by the AMOS program in the Department of Energy (DOE) Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division. MeV-UED is operated as part of the Linac Coherent Light Source at the SLAC National Accelerator Laboratory, supported in part by the DOE Office of Science, Office of Basic Energy Sciences, SUF Division Accelerator and Detector R&D program, the LCLS Facility, and SLAC. Study coauthor David Sanchez was supported by Lawrence Livermore National Laboratory.
Journal
Nature Communications
DOI
10.1038/s41467-023-38513-6
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Rehybridization dynamics into the pericyclic minimum of an electrocyclic reaction imaged in real-time
Article Publication Date
18-May-2023




