RIVERSIDE, Calif. – Toxoplasma gondii, a protozoan parasite about five microns long, infects a third of the world’s population. Ingested via undercooked meat or unwashed vegetables, the parasite infects 15-30 percent of the US population. In France and Brazil, up to 80 percent of the population has the infection.
Particularly dangerous during pregnancy – infection in pregnant women can cause serious congenital defects and even death of the fetus – this chronic infection has two components: the unicellular parasite, and inflammation of tissues it causes.
Working on mice (like all mammals, a natural host for this parasite), a University of California, Riverside team of biomedical scientists reports in the journal PLOS Pathogens that Toxoplasma infection leads to a disruption of neurotransmitters in the brain and postulates that it triggers neurological disease in those already predisposed to such a disease.
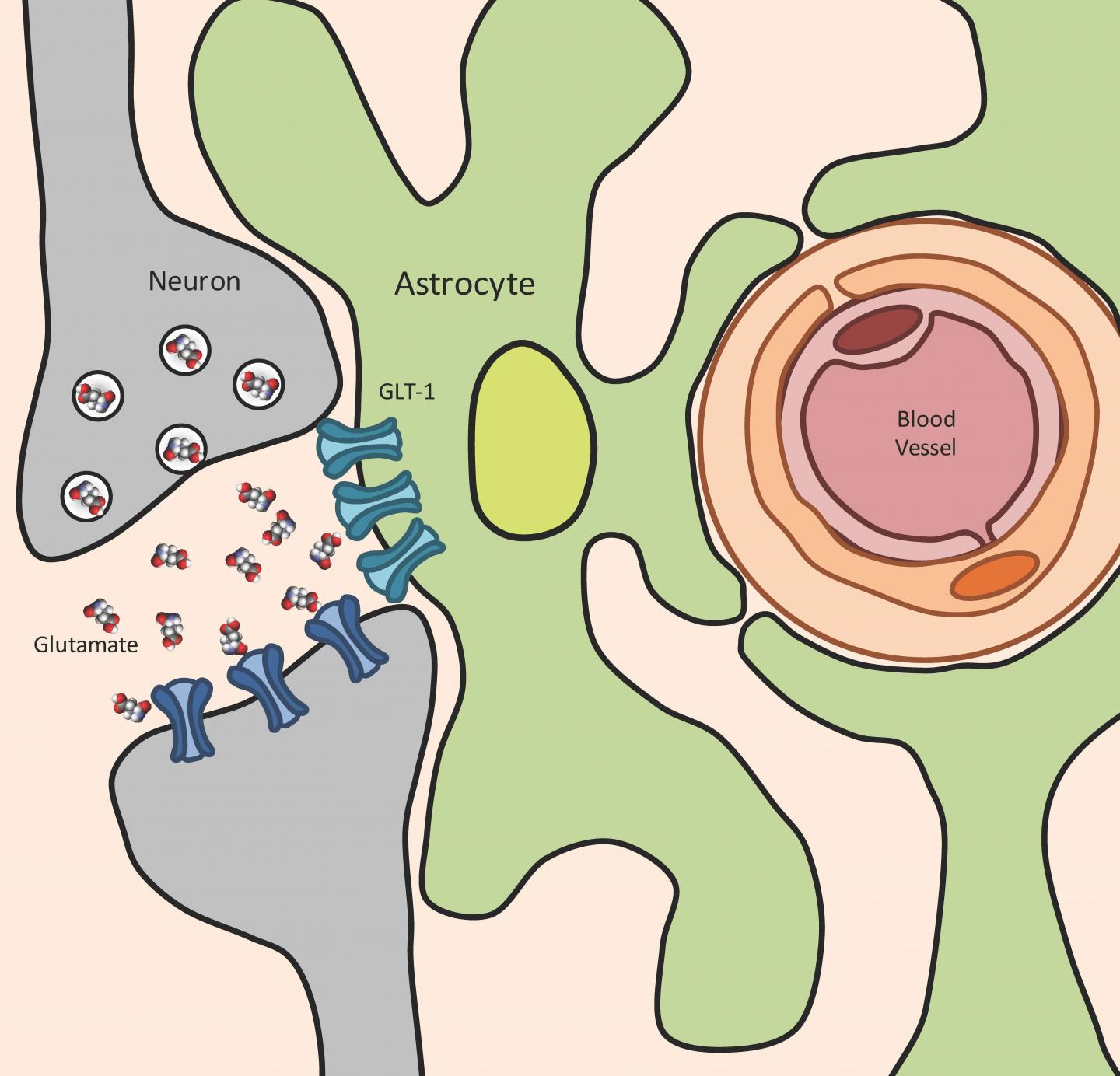
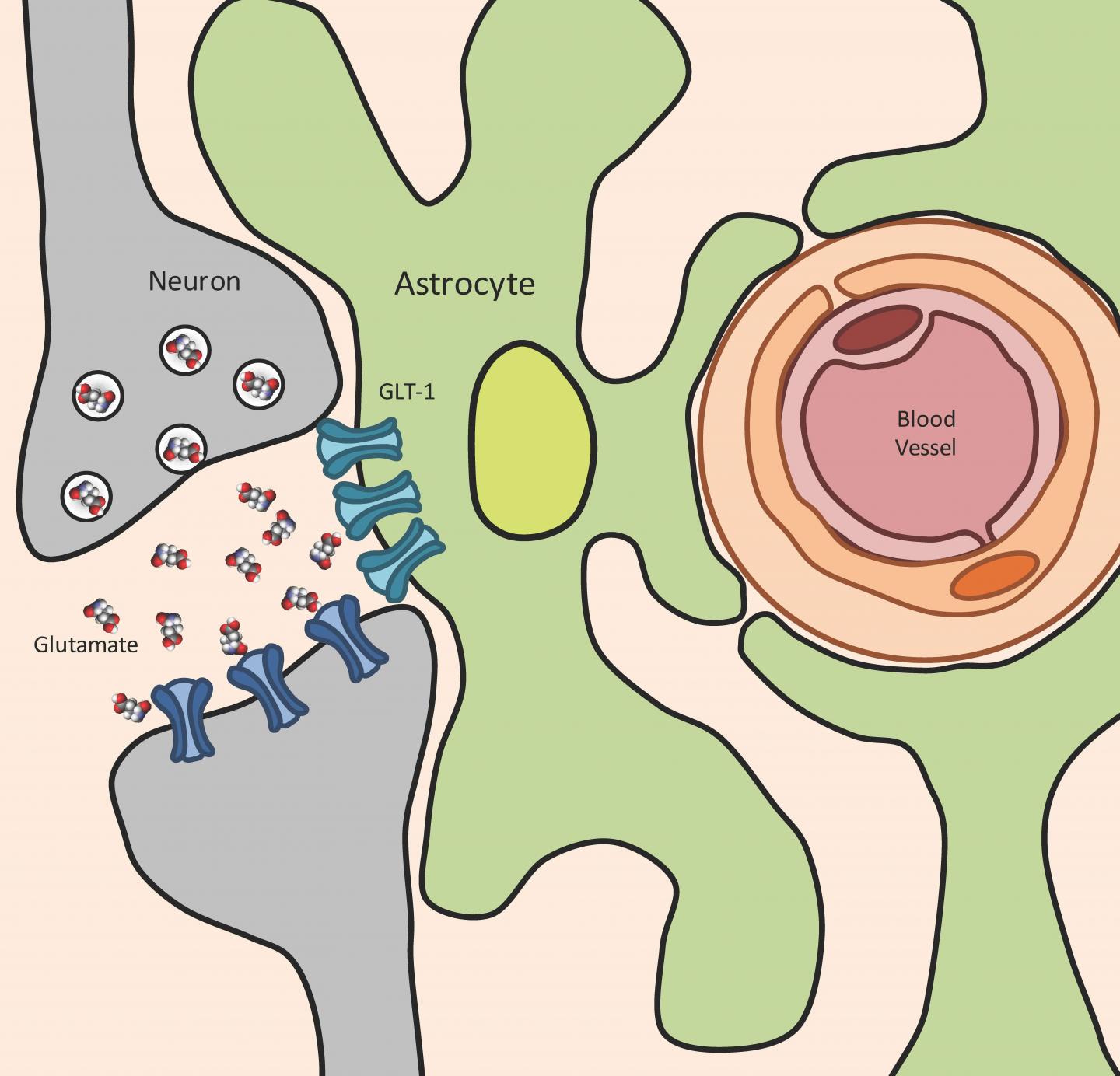
They note that Toxoplasma infection leads to a significant increase in glutamate – the primary and most important neurotransmitter in the brain, which transmits excitatory signals between neurons. This glutamate increase is “extracellular,” meaning outside the cell, and is strictly controlled by specialized cells in the central nervous system (brain and spinal cord), called astrocytes. Glutamate buildup is seen in traumatic brain injury as well as highly pathological and neurodegenerating diseases such as epilepsy, multiple sclerosis and amyotrophic lateral sclerosis (ALS).
One role astrocytes play is to remove extracellular glutamate, lest it increase to pathological levels that could damage neurons. This is primarily achieved using a glutamate transporter, called GLT-1, tasked with regulating extracellular glutamate. GLT-1 soaks up glutamate released by neurons and converts it back into the safer substance glutamine, which can then be used by cells for energy.
“When a neuron fires it releases glutamate into the space between itself and a nearby neuron,” explained lead researcher Emma H. Wilson, an associate professor in the Division of Biomedical Sciences in the School of Medicine, who has worked on toxoplasmosis for more than 15 years. “The nearby neuron detects this glutamate which triggers a firing of the neuron. If the glutamate isn’t cleared by GLT-1 then the neurons can’t fire properly the next time and they start to die.”
Wilson and her team found that during toxoplasma infection, astrocytes swell and are not able to regulate extracellular glutamate concentrations. Further, GLT-1 is not expressed properly. This leads to a buildup of the glutamate released from neurons and the neurons misfire.
“These results suggest that in contrast to assuming chronic Toxoplasma infection as quiescent and benign, we should be aware of the potential risk to normal neurological pathways and changes in brain chemistry,” Wilson said.
When the researchers treated the infected mice with ceftriaxone, an antibiotic known to produce beneficial results in mouse models of ALS as well as neuroprotection in a variety of central nervous system injuries, they found that GLT-1 was upregulated. This restoration of GLT-1 expression significantly reduced extracellular glutamate from pathological to normal concentrations, returning neuronal function to a normal state.
“We have shown for the first time the direct disruption of a major neurotransmitter in the brain resulting from this infection,” Wilson said. “More direct and mechanistic research needs to be performed to understand the realities of this very common pathogen.”
Next, Wilson and her colleagues will research what initiates the downregulation of GLT-1 during chronic Toxoplasma infection.
“Despite the importance of this transporter to maintaining glutamate homeostasis, there is little understanding of the mechanism that governs its expression,” Wilson said. “We’d like to know how cells, including peripheral immune cells, control the parasite in the brain. Toxoplasma infection results in the lifelong presence of parasitic cysts within the neurons in the brain. We’d like to further develop a project focused on killing the cysts, which is where the parasite hides from the immune response for the rest of the infected person’s life. Getting rid of the cyst removes the threat of reactivation of the parasite and the risk of encephalitis while also allowing us to minimize chronic inflammation in the brain.”
Mysteriously, the parasite that causes toxoplasmosis can sexually reproduce only in cats. Asexually, it can replicate and live in any mammalian cell that has a nucleus. Indeed, the parasite has been found in every mammal ever tested.
Post-infection, a competent immune system is needed to prevent parasite reactivation and encephalitis. Infected people with compromised immune systems need to be on prophylactic drugs for life. Otherwise they are at risk of cyst reactivation and death. The parasite lives in areas of the brain that have the potential to disrupt certain behaviors such as risk-seeking (infected mice will run toward cat urine instead of away from it).
The parasite is not as latent or dormant as researchers once thought. Cases of congenital infection and retinal toxoplasmosis are on the rise (the brain and retina are closely linked). People who have schizophrenia are more likely to be infected with Toxoplasma. Infection shows some correlation with Alzheimer’s disease, Parkinson’s disease and epilepsy.
Nevertheless, Wilson notes that infection is no cause for major worry.
“We have been living with this parasite for a long time,” she said. “It does not want to kill its host and lose its home. The best way to prevent infection is to cook your meat and wash your hands and vegetables. And if you are pregnant, don’t change the cat litter.”
###
The research was supported by grants from the National Institutes of Health and UC Riverside’s Academic Senate; the Office of Research and Economic Development; and the Graduate Division.
Wilson was joined in the study by her former graduate student Clement N. David (first author of the research paper), now at the University of North Carolina; Elma S. Frias, Jenny I Szu, Philip A. Viera, Jacqueline A. Hubbard, Jonathan Lovelace, Marena Michael, Danielle Worth, Kathryn E. McGovern, Iryna Ethell, B. Glenn Stanley, Edward Korzus, Todd A. Fiacco and Devin Binder – all at UC Riverside.
The University of California, Riverside (http://www.ucr.edu) is a doctoral research university, a living laboratory for groundbreaking exploration of issues critical to Inland Southern California, the state and communities around the world. Reflecting California’s diverse culture, UCR’s enrollment has exceeded 21,000 students. The campus opened a medical school in 2013 and has reached the heart of the Coachella Valley by way of the UCR Palm Desert Center. The campus has an annual statewide economic impact of more than $1 billion. A broadcast studio with fiber cable to the AT&T Hollywood hub is available for live or taped interviews. UCR also has ISDN for radio interviews. To learn more, call (951) UCR-NEWS.
Media Contact
Iqbal Pittalwala
[email protected]
951-827-6050
@UCRiverside
http://www.ucr.edu
The post Scientists unpack how Toxoplasma infection is linked to neurodegenerative disease appeared first on Scienmag.