Organometallic compounds, molecules made up of metal atoms and organic molecules, are often used to accelerate chemical reactions and have played a significant role in advancing the field of chemistry.
Credit: Takebayashi et al., 2023
Organometallic compounds, molecules made up of metal atoms and organic molecules, are often used to accelerate chemical reactions and have played a significant role in advancing the field of chemistry.
Metallocenes, a type of organometallic compound, are known for their versatility and special “sandwich” structure. Their discovery was a significant contribution to the field of organometallic chemistry and led to the awarding of the Nobel Prize in Chemistry in 1973 to the scientists who discovered and explained their sandwich structure.
The versatility of metallocenes is due to their ability to “sandwich” many different elements to form a variety of compounds. They can be used in various applications, including the production of polymers, glucometers – used to measure the amount of glucose in the blood, perovskite solar cells, and as a catalyst, a substance that increases the rate of a chemical reaction without being consumed or changed by the reaction itself.
Dr. Satoshi Takebayashi, a researcher at the Science and Technology Group at the Okinawa Institute of Science and Technology (OIST), together with Dr. Hyung-Been Kang, a scientist at OIST’s Engineering Section, and scientists from Germany, Russia, and Japan, has successfully developed a new metallocene compound at OIST.
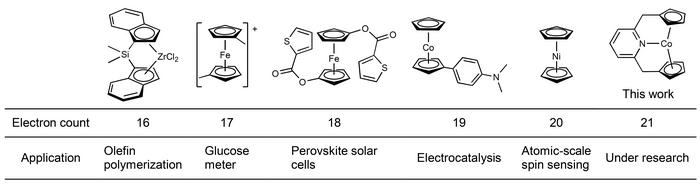
The chemical structure of metallocenes can accommodate a variety of electron counts, allowing for the formation of complexes with up to 20 electrons. However, the 18-electron structure is most favored as it is the most stable version.
“Having more than 18 electrons is known to be rare because if you deviate from 18, the chemical bonds of the metallocenes start to elongate, break, and change structure. However, we added two more electrons to a 19-electron metallocene and created a 21-electron metallocene. I think most people didn’t think this was possible, but our 21-electron metallocene is stable in solution and solid states and can be stored for a long time,” Dr. Takebayashi explained.
With this new metallocene, we can potentially create novel materials that can be used for applications in medicine, catalysis, and the energy sector, helping to solve important global problems and improving our quality of life.
Because the sandwich structure of metallocenes can easily be altered, the most challenging part of the research was for the scientists to show that the nitrogen had successfully bonded to the cobalt without altering the sandwich structure. They had to rigorously prove that the metallocene was properly bonded to all neighboring carbon atoms and that the nitrogen atom was attached to the cobalt atom. To do this, Dr. Takebayashi organized a strong team of researchers with different specialties and unambiguously showed that all the elements had bonded well.
“This breakthrough would not have been possible without the participation of my collaborators who did substantial work,” Dr. Takebayashi added. Dr. Satoshi Takebayashi, Jama Ariai, Dr. Urs Gellrich, Sergey Kartashov, Dr. Robert Fayzullin, Dr. Hyung-Been Kang, Dr. Takeshi Yamane, Dr. Kenji Sugisaki, and Prof. Kazunobu Sato have coauthored an article published in the journal Nature Communications detailing their discovery.
Dr. Takebayashi’s future research will focus on the utilization of the 21-electron metallocene for more applicable science such as catalysis and material science, as well as the discovery of unprecedented organometallic chemistry based on this finding.
Journal
Nature Communications
DOI
10.1038/s41467-023-40557-7
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Synthesis and characterization of a formal 21-electron cobaltocene derivative
Article Publication Date
5-Sep-2023




