Credit: Dmitry Bratanov et al./Nature Communications
The structure of an Organic Lake Phycodnavirus rhodopsin II (OLPVRII), which is a unique protein found in the genome of giant viruses, has been determined thanks to the work of MIPT graduates and PhD students. The paper was published in Nature Communications.
The study is the result of a collaboration that included many MIPT alumni. Dmitry Bratanov was among them. Dmitry, who currently works at the Institute of Complex Systems (ICS-6) at the Research Center Juelich, Germany, says that although viral rhodopsins were first discovered in the so-called giant viruses several years ago, their structure, function, and biological role have remained unclear until now.
A giant virus is a very large virus, the size of a typical bacterium. It is so big that it is visible under a light microscope. Giant viruses infect green algae, which produce oxygen and help maintain the natural ecological balance of the world’s ocean. Therefore, giant viruses are of considerable research interest from an environmental perspective.
“In this work, we deciphered the high-resolution structure of OLPVRII, functionally characterized the protein, and showed that it forms pentamers not only in crystals but in lipid membranes as well,” explains Dmitry. “This was no easy task. Numerous experiments had to be performed, for some of them we used sophisticated techniques and equipment. What we have achieved is the result of the hard and meticulous work of the international group of scientists.”
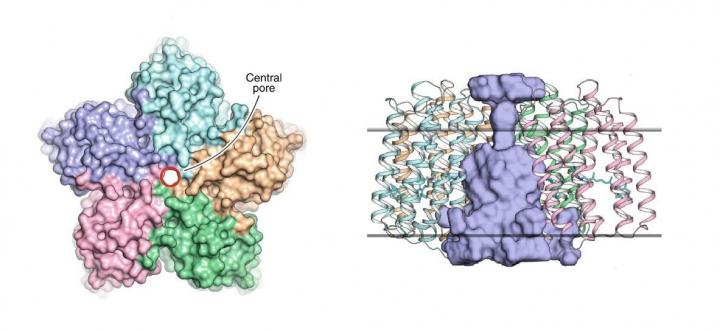
This pentameric organization has previously been observed in some other rhodopsins, such as, for instance, in the light-driven sodium pump KR2. However, what makes the OLPVRII structure peculiar is that it has an unusual pore in the center (see figure 1). Its function remains unknown.
“We think that perhaps the pore acts as an ion channel, most probably for chloride ions,” says Kirill Kovalev, a co-author of the paper and PhD student at MIPT.
Ion channels are proteins that create a passive pathway for ions to flow across the cell membrane. These channels are usually closed in the dark, meaning ions cannot move into or out of the cell. In the case of light-sensitive channels, they open in response to light absorption, which allows ions to flow down the concentration gradient. In other words, ions move in the direction that would even out the concentrations of ions inside and outside the cell.
A typical example of a light-sensitive channel is channelrhodopsin 2. It was found in the green alga Chlamydomonas reinhardtii and is widely used as an optogenetic tool. As for OLPVRII, the researchers think that this might be the first pentameric light-gated ion channel ever discovered, as suggested by the determined structure and molecular simulations.
“However, the channel activity of OLPVRII has yet to be demonstrated,” notes Kirill Kovalev.” We will continue our research and will definitely find out why this unusual rhodopsin was created by nature. Perhaps it helps the host continue to carry out its life-sustaining functions when a cell is infected by a virus, or maybe it is a sensor.”
That said, studying the structure of the viral rhodopsin shed some light on how it functions. It was shown that OLPVRII, like most other rhodopsins, acts as a proton pump. This however is unlikely to be its principal function, the researchers say. Its main purpose has yet to be investigated and proved.
“If we prove that this viral rhodopsin is in fact an ion channel, it can become a great tool in optogenetics and biomedical applications,” says paper co-author Valentin Gordeliy, who leads research groups at the Institute of Structural Biology in Grenoble and the Research Center Juelich. Valentin is also a research coordinator at MIPT’s Research Center for Molecular Mechanisms of Aging and Age-Related Diseases.
The researchers say that the new tool will outperform all its counterparts thanks to the advantages of its pentameric structure: the ease with which you can genetically manipulate the properties of the protein and perhaps the high currents circulating through the central pore.
To have the priority right to utilize their invention, the authors of the paper have submitted a patent application for the use of the viral rhodopsin OLPVRII in the field of optogenetics.
Optogenetics is a branch of biophysics that uses light to control cells in a living organism. As has already been demonstrated, optogenetics can be applied to restore vision and hearing loss, help control movement in neurological patients, and treat those with Parkinson’s and Alzheimer’s disease.
According to Valentin Gordeliy, MIPT has all the necessary equipment required to conduct a detailed study of the functions of the viral rhodopsin. The group will continue their research into OLPVRII, which will be of great significance for biology, evolutionary science, optogenetics, and ecology.
###
Media Contact
Varvara Bogomolova
[email protected]
7-916-147-4496
Original Source
https:/
Related Journal Article
http://dx.




