A team of scientists from the University of Ottawa have opened a window into the cause of a rare genetic disorder that causes mortality in young children
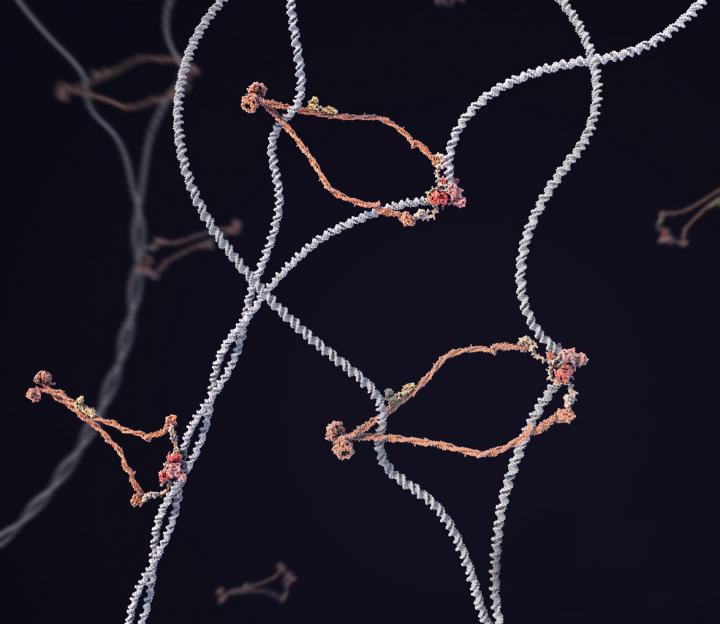
Credit: D’Amours Lab/University of Ottawa
Damien D’Amours and his team at the Ottawa Institute of Systems Biology needed three years to discover the molecular defects associated with the LIC Syndrome, a serious genetic disorder that affects young children and result in acute respiratory distress, immune deficiency and abnormal chromosomes.
Onset of symptoms occurs in the first few months after birth in infants suffering from Lung disease Immunodeficiency and Chromosome breakage (LIC). Typically, patients experience failure to thrive and immune deficiency, which can eventually progress to fatal pediatric pulmonary disease in early childhood. The disease is caused by small inactivating mutations in NSMCE3, a gene encoding an essential factor found in the nucleus of human cells.
This research represents one of the most important milestones in developing treatments to improve the lives of LIC syndrome patients. Damien D’Amours is a Full Professor in the Department of Cellular & Molecular Medicine of the Faculty of Medicine whose lab is focused understanding the mechanisms used by cells to promote efficient cell division and proliferation. He provided further insights into the study’s findings.
What exactly have you discovered?
“We discovered how defects in a “DNA compaction machine” within our cells can cause a rare genetic disorder that kills young children (i.e., the LIC syndrome). We found the molecular cause by using an exciting mix of biophysics, advanced genetics and classical biochemistry to demonstrate that an enzyme has the rare ability to compact DNA within our cells.”
How did you do it?
“We developed a completely novel system to purify a human enzyme that nobody in the world has ever successfully purified – the “Smc5/6 complex.” The Smc5/6 complex is a crucial effector of chromosome integrity, and our breakthrough allowed us to reveal the structure of the enzyme and its powerful ability to compact DNA structure in space. We then modelled the mutations causing the LIC syndrome in our system and showed that the mutations affect ability of the Smc5/6 complex to repair chromosomes in cells, thus explaining how LIC mutations affect the ability of cells to maintain healthy genomes.”
You used the “systems biology” approach to reach your conclusions; please explain this.
“The advent of systems biology has revolutionized biomedical research in recent years. This approach relies on the use of integrative “omics” technologies and model organisms to provide a systems-level understanding of human diseases. (Omics is a general term to describe “large-scale genomics, proteomics, and metabolomics technologies.”) The University of Ottawa has been at the forefront of this revolution in research with the creation of the Ottawa Institute of Systems Biology (OISB). We took advantage of the systems biology approach to develop completely new systems to purify an enzyme never purified before. Then we used innovative mix of biophysics, proteomics and classical biochemistry to reveal the mode of action of the Smc5/6 complex and how mutations in this complex can cause severe defects in DNA repair.”
Why is this an important find?
“My research team and our collaborators are performing research at the absolute cutting-edge of our field and, as the leading laboratory on this project, we feel our research represents one of the most important milestones on the way to devise treatments for LIC syndrome patients. Prior to our work, nobody knew the biochemical cause for the LIC syndrome and how the enzyme mutated in this disease might affect the cells of patients/children; we provided answers to these fundamental questions.”
###
Media Contact
Paul Logothetis
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




