Credit: FWC Fish and Wildlife Research Institute
A team of scientists from Tyumen together with colleagues found and described previously unknown tapeworm proteins that suppress the activity of trypsin and efficiently protect the parasites from being digested inside a host’s intestinal tract. The analogs of these proteins are found in many other living organisms and were described in some other parasite worms. The results of the study were published in the Molecular & Biochemical Parasitology journal.
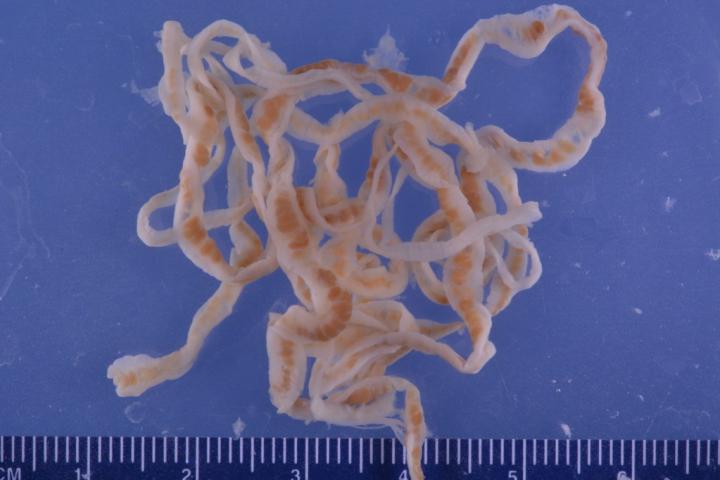
Tapeworms or cestodes are a class of flat parasite worms that usually have several hosts during their life cycle. The adults live in the intestinal tracts of the vertebrates and may pose a considerable threat to human and animal health. Due to their parasitic mode of life these worms completely lost their digestive apparatus but have a well-developed reproductive system and special organs that help them attach to the host’s tissues. They also needed a mechanism to protect themselves from intestinal substances, specifically from digestive enzymes. One of such enzymes called trypsin breaks down proteins.
“There are lots of studies describing the inhibitors (proteins that block the activity of digestive ferments) of nematode worms and covering numerous species of these parasites, including the well-known ascarides. However, few works address the biochemistry of cestodes, and their molecular diversity is only superficially studied. The researchers of tapeworms traditionally paid attention mainly to tenias and echinococci, as they are the most dangerous for humans and animals. Other species remained understudied, and neither their inhibitors nor the mechanisms of their work have been known until recently”, said Eugene Rogozhin, PhD (Bioorganic Chemistry), and a senior researcher at Tyumen State University.
The team studied the Triaenophorus nodulosus worms. These parasites are the cause of triaenophorosis — a dangerous disease leading to mass extinction of young fish in certain freshwater species. The worms were produced from the intestines of a common pike caught in the Rybinsk Reservoir. The proteins obtained from the homogenate of the cestodes were divided into fractions using the liquid chromatography methods. After that the fractions that were the most effective in inhibiting digestive enzymes were selected. The molecular mass of the inhibitors was determined using polyacrylamide gel electrophoresis (a method based on the differences in the mobility of molecules with different sizes in a gel under the influence of an electric field). The scientists managed to identify two previously unknown polypeptides (around 14.4 kDa in mass) with different N-terminal amino-acid residues. After searching for homologous sequences the team concluded that the peptides belonged to their own type of trypsin inhibitors similar to Kunitz-type proteins that are found both in in- and vertebrates. Besides their inhibition activity, these ferments also play a role in blood clotting and inflammation processes. Proteins of the same type had been previously obtained from other tapeworm species, in which they also weakened the host’s immune resistance.
###
The members of the team also represented Papanin Institute for the Biology of Inland Waters of the Russian Academy of Sciences, Institute of Bioorganic Chemistry of the Russian Academy of Science, Gause Institute of New Antibiotics, Institute of Systematics and Ecology of Animals of the Siberian Brunch of the Russian Academy of Sciences, and Tomsk State University.
Media Contact
Olga Chirkova
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




