Azide compounds play a pivotal role for subsequent synthesis of organonitrogens such as amines and triazoles that are essential compounds in organic and materials chemistry. Triazoles that can be synthesized by the ‘click’ reaction have attracted attention in the development of pharmaceuticals and other industries. However, the azido groups are electrophilic and are susceptible to various nucleophiles such as carbanions. This poses a significant challenge for the synthesis of carbanions having azido groups.
Credit: Suguru Yoshida from Tokyo University of Science
Azide compounds play a pivotal role for subsequent synthesis of organonitrogens such as amines and triazoles that are essential compounds in organic and materials chemistry. Triazoles that can be synthesized by the ‘click’ reaction have attracted attention in the development of pharmaceuticals and other industries. However, the azido groups are electrophilic and are susceptible to various nucleophiles such as carbanions. This poses a significant challenge for the synthesis of carbanions having azido groups.
To this end, a team of researchers from Japan, led by Associate Professor Suguru Yoshida from Tokyo University of Science (TUS), has now developed an innovative and efficient azide synthesis method. In their recent article published in the journal Frontiers in Chemistry, Dr. Yoshida and his colleagues Ms. Rina Namioka (TUS) and Ms. Minori Suzuki (Tokyo Medical and Dental University) have detailed an efficient method to prepare organomagnesium (organic compound containing a magnesium ion linked to a carbon) intermediates having a protected azido group. This paper was published in Volume 11 of the journal Frontiers in Chemistry.
“We conducted this research because we believe that the range of azide compounds that can be easily synthesized can be expanded by developing a synthesis method based on the azide group protection method that we discovered,” says Dr. Yoshida.
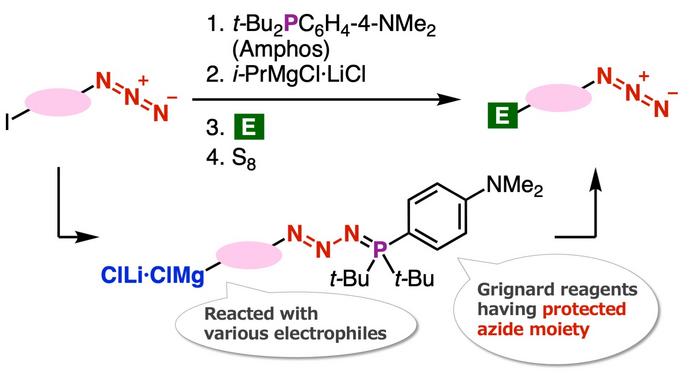
The novelty of this synthesis method lies in protection of azido groups with di(tert-butyl)(4-(dimethylamino) phenylphosphine (Amphos) and following iodine-magnesium exchange realized the preparation of organomagnesium intermediates, which served in the synthesis of diverse azides by transformations with various electrophiles followed by deprotection with elemental sulfur. In this study, the authors found a new azide synthesis method utilizing the Grignard reaction and developed a new method for the synthesis of 1,2,3-triazoles.
Specifically, the team found that the iodine-magnesium exchange reaction proceeds efficiently with aryl iodides having azido groups by using the ‘azide group protection method’ that they have recently developed. Speaking about the underlying mechanism that enabled the researchers to achieve this novel azide synthesis method, Dr. Yoshida recalls, “The Grignard reaction of organomagnesium compounds that were synthesized by this reaction succeeded in the synthesis of a wide range of azide compounds.”
As azide compounds play a central role in the synthesis of 1,2,3-triazoles in click chemistry, this method is expected to contribute to the efficient development of pharmaceuticals and other industrially important products.
“Our lab is currently conducting research on the preparation and transformation of carbanions with phosphazide moieties to expand the toolbox of such compounds,” notes an optimistic Dr. Yoshida. The seamless, efficient synthesis of azides facilitating subsequent synthesis of a broad range of organonitrogen compounds such as amines and triazoles will immensely benefit synthetic organic chemistry, pharmaceutical sciences, and materials chemistry communities.
***
Reference
DOI: https://doi.org/10.3389/fchem.2023.1237878
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society”, TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Associate Professor Suguru Yoshida from Tokyo University of Science
Dr. Suguru Yoshida is an Associate Professor in the Faculty of Advanced Engineering, Department of Biological Science and Technology, Tokyo University of Science, Japan. He received his B.S. and M.S. degrees from the University of Tokyo. After receiving his Ph.D. in engineering from Kyoto University (2009), he has served as a post-doctoral fellow (Kyushu University and University of Hawaii at Manoa), an Associate Professor (Tokyo Medical and Dental University), Program Officer, MEXT prior to his current role. He has won multiple awards including Thieme Chemistry Journals Award, the Young Scientists’ Prize, and the Commendation for Science and Technology by MEXT. He has published over 130 articles garnering over 3600 citations in his primary research area of synthetic organic chemistry and chemical biology.
Funding Information
This study was supported by JSPS KAKENHI Grant Number JP22H02086 and Asahi Glass Foundation.
Journal
Frontiers in Chemistry
DOI
10.3389/fchem.2023.1237878
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Synthesis of 1,2,3-triazoles using Grignard reactions through the protection of azides
Article Publication Date
31-Jul-2023
COI Statement
The authors declare no competing financial interest




