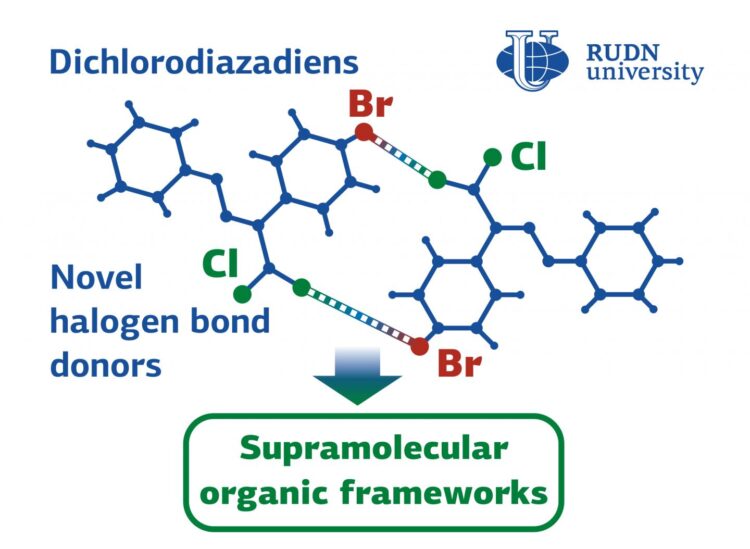
RUDN University chemists derived molecules that can assemble into complex structures using chlorine and bromine halogen atoms. They bind to each other as “velcro” – chlorine “sticks” to bromine, and vice versa. As a result supramolecules are assembled from individual molecules. The obtained substances will help to create supramolecules with catalytic, luminescent, conducting properties. The study is published in Mendeleev Communications.
Supramolecules are the structures made of several molecules. Individual molecules are combined, for example, by self-assembly or without external control. The resulting structure has properties that the molecules did not have individually. That is the way to create new materials, catalysts, molecular machines for drug delivery, conductors, and so on. To get a material with the specified properties, you need to choose the right starting molecules and auxiliary substances that will ensure their unification. One of the ways to control self-assembly is halogen-halogen interactions. These are the chemical bonds forming between two halogens (for example, chlorine, fluorine, bromine). RUDN University chemists have created a molecule with a halogen bond that can form supramolecules by itself or provide the required self-assembly with other substances. They will help to create substances for the chemical industry, medicine, and electronics.
“The possibility of fine control of the local molecular environment is highly desirable to access new properties for substances that function as catalysts, luminescent or conductive materials, etc. 2-4 Halogen bonding has recently emerged as useful instrument for the accurate control of the structural organization of such supramolecular materials. In this context, halogen-halogen interactions received a particular attention and were intensively explored both experimentally and theoretically”, said the authors of the article.
Chemists used 7 types of hydrazones and carbon tetrachloride as starting materials for synthesis. The reaction lasted 1-3 hours at room temperature, with copper chloride as a catalyst. As a result, 7 compounds were obtained, two of them were suitable for the formation of a halogen-halogen bond between themselves or with other substances. RUDN University chemists studied them with X-ray diffraction analysis. Then the scientists built a 3D model of intramolecular interactions and confirmed their observations using topological analysis of the electron density distribution.
Thanks to the ability to form halogen-halogen bonds, new substances can control the self-assembly of molecules or form supramolecules themselves. That is because the new substances contain atoms of two halogens on two sides of the molecules — chlorine and bromine. As a result, they can connect to each other through halogens — chlorine combines with bromine, and vice versa. They can also form halogen-halogen bonds with other substances, thus controlling the assembly of supramolecules.
“Calculations demonstrated that highly polarizable dichlorodiazadiene unit is capable of acting as a relatively strong halogen bond donor. When the dye was decorated with halogen bond-accepting bromine atoms, formation of 3D supramolecular framework was observed”, said the authors of the article.
###
Media Contact
Valeriya Antonova
[email protected]
Related Journal Article
http://dx.