Credit: Alexey Festa
Researchers from RUDN University conducted an effective three-component reaction, obtaining unusual organic compounds. The latter are structurally similar to a number of biologically active compounds – which makes it possible to use them in pharmaceutics (for example, as antitumor drugs and agents for Alzheimer's disease). The results of the work are presented in the journal Mendeleev Communications.
Organic compounds are widely used as medicines, agrochemicals, flavoring agents and preservatives. They are also employed in the creation of new materials, electronic devices and many other objects. Usually the synthesis of organic compounds is carried out sequentially. The raw materials available in industrial quantities are modified gradually: functional groups are introduced or changed, the scaffold is expanded, the structure is getting more complicated. This approach implies the isolation and purification of all intermediate compounds on the way to the target molecule.
Nowadays, domino and multicomponent reactions, as well as one-pot processes (when all the multistage synthesis goes in one vessel) are actively used and developed by chemists. Domino reactions are interesting because they automatically follow one after another in one vessel without the addition of additional reagents. "The reactions of these three types are convenient means for the effective and environmentally friendly synthesis of organic substances. They allow to avoid the isolation of intermediates, and, accordingly, save solvents and sorbents, reduce labor costs, cut on the amount of waste", – said Alexey Festa, the lead author of the paper.
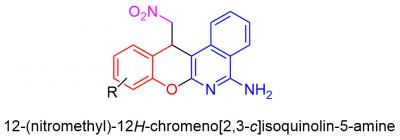
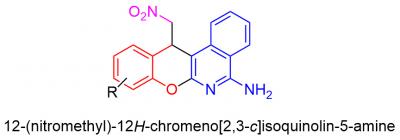
In the research carried out by organic chemists of RUDN University, the three-component reaction of α-cyano-o-tolunitrile with salicylic aldehydes and C-H acid was studied for the first time. They found out that carrying out the reaction in a single-reactor mode allows the production of complex heterocyclic compounds – nitromethyl-substituted chromoisoquinolinamines – with a good yield. The process is very efficient – two new carbon-carbon bonds, one oxygen-carbon and one nitrogen-carbon are formed for one synthetic operation, and two cycles are closed.
The compounds obtained by RUDN University scientists are interesting by the fact of their structural similarity with a number of biologically active compounds. For example, the chromopyridine fragment can be found in pranoprofen, a popular eye care drug, as well as chromenoktrine (CT6) — a potential remedy for Alzheimer's disease. The 2-aminochrome fragment is found in crolibulin (EPC2407), a substance that exhibits antitumor activity and is now undergoing a stage II clinical trial. All this allows us to believe that studying the biological properties of these compounds will also lead to the discovery of useful activity.
"Precisely these structural similarities make it possible to use synthesized substances in pharmaceuticals. What does it mean? Medications usually work by binding to a biological targetin the human body –a receptor, for instance. If it is known that a certain chemical substance has a certain biological activity, we can anticipate resembling compounds to exhibit the same activity. In other words, structural similarity is enough to start biological tests", — Festa explained.
Finally, the importance of the work lies in the fact that the chemists have shown that it is possible to use such an easily accessible reagent as α-cyano-o-tolunitrile as a dinitrile component in domino reactions, which opens the door to the synthesis of a huge number of other compounds. In the futurethe researchers will expand the synthetic potential of the studied reaction and show the generality of the approach on the use of other ?-? acids and nucleophiles.
###
Media Contact
Valeriya V. Antonova
[email protected]
http://eng.rudn.ru/
Related Journal Article
http://dx.doi.org/10.1016/j.mencom.2017.09.006