Biomedical engineers at Duke University have recently made significant strides in the field of synthetic biology, presenting an innovative approach that enhances the capability of bacteria to synthesize specific proteins. This ingenious method not only boosts the production of proteins essential for various industrial applications, but it also enables bacteria to produce proteins that are typically hostile to their survival, including antibiotics. This breakthrough has the potential to transform biotechnology by optimizing the way microorganisms are utilized in manufacturing processes.
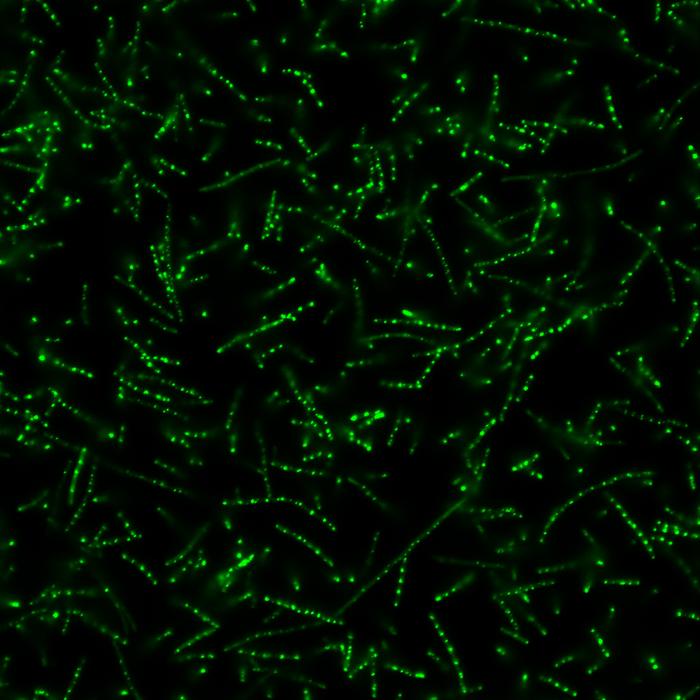
At the core of this advancement is a novel technique that directs bacteria to create synthetic disordered proteins, which aggregate to form what are known as biological condensates. These condensates act as specialized compartments within the cell, effectively trapping messenger RNA (mRNA) that contains instructions for protein synthesis. By clustering both the mRNA and the necessary machinery for its translation, biological condensates markedly increase the efficiency of protein production within the bacterial cell.
The implications of this research extend to various industries reliant on bacterial production systems, including pharmaceuticals, industrial chemicals, and biofuels. As the demand for sustainable and efficient production processes continues to rise, this technique could offer a powerful solution for manufacturers seeking to optimize their microbial hosts. The findings from these experiments shed light on the functional versatility of biological condensates, which are already known to exist in natural systems, suggesting that engineers can harness and manipulate them to enhance cellular performance.
Biological condensates play a crucial role in cellular processes by allowing cells to temporally control gene expression in response to external stimuli. By regulating gene expression at the level of protein production, cells can adapt more rapidly to changing conditions. This rapid response is critical for survival, especially under adverse environmental circumstances. However, engineering these condensates for specific purposes has proven to be complex due to the intricacies of their structure and function in living cells.
The research team at Duke, guided by Ashutosh Chilkoti, a highly esteemed Professor of Biomedical Engineering, has broken new ground by fabricating synthetic versions of biological condensates. This accomplishment allows for greater control over the properties and functions of these condensates, tailoring them to suit the researchers’ objectives. As Daniel Shapiro, a PhD student in Chilkoti’s lab, articulates the significant progress made, this marks an encouraging step towards the reprogramming of living systems in remarkably novel ways.
Specifically, the Duke laboratory specializes in the study of elastin-like polypeptides (ELPs), which are long, disordered proteins capable of responding to environmental factors such as temperature and pH levels. Thanks to their unique characteristics, these polypeptides can be designed to either cluster together or disperse, allowing for manipulation of their behavior within the cell. This flexibility presents an exciting opportunity for biotechnological applications, helping to create engineered systems that can achieve specific, desired outcomes.
In previous research, the Chilkoti laboratory successfully demonstrated that ELPs could be programmed within bacterial cells to generate synthetic disordered proteins that form condensates. This was a major breakthrough that paved the way for the current exploration into directing cells to produce proteins with more specificity and speed. The researchers built upon their earlier findings to develop a new methodology that instructs bacteria not only to create ELPs, but also to bind them with specific RNA sequences.
By concentrating these RNA sequences—vital for the synthesis of proteins—within the biological condensates, the team significantly raised their availability to the cellular machinery responsible for translation. This interaction essentially serves as a catalyst, increasing the rate at which proteins are produced in bacterial cells, thus enhancing overall productivity and efficiency. Shapiro emphasizes the unprecedented ability to concentrate RNA in this manner, allowing for an expedited translation process that promises to reshape bacterial production scenarios.
As the team forges ahead with this groundbreaking research, they continue to refine the features of their synthetic biomolecular condensates. Preliminary experiments indicate an interesting relationship between the viscosity of the condensates and protein output; specifically, more viscous condensates tend to produce lower yields of proteins. This discovery provides valuable insights and levers for researchers to manipulate the system further, enabling them to find optimal conditions for protein production.
This research has potentially profound implications for two major sectors: biopharmaceuticals and antibiotic manufacturing. Presently, many biological therapeutics such as antibodies and vaccines require mammalian cells for production due to their specialized biochemical machinery. However, advancements in synthetic condensates could allow bacteria to function more effectively in this domain by clustering the necessary molecular components for efficient therapeutic production. Furthermore, RNA-guided condensates may help encase proteins in a safe manner, addressing a common problem faced in antibiotic synthesis where the products can be detrimental to the host cells.
This impressive research endeavor, which appeared online on February 10, 2025, in the prestigious journal Nature Chemistry, marks a significant milestone in the ongoing exploration of synthetic biology and its potential to revolutionize industrial biotechnology. The future holds promising possibilities as researchers like Shapiro and Chilkoti continue to probe into the molecular designs that can reshape and optimize living systems for advantageous outcomes.
The research findings received robust support from the Air Force Office of Scientific Research as well as the National Institutes of Health, corroborating the importance of these advancements not only for academic curiosity but also for practical applications in various industrial settings. As the scientists refine their methods and explore new avenues, the potential for synthetic biomolecular condensates to alter the landscape of protein engineering remains vast and exciting, cementing their place in the annals of modern biotechnology.
Subject of Research: Proteins synthesis in bacterial cells
Article Title: Synthetic biomolecular condensates enhance translation from a target mRNA in living cells
News Publication Date: 10-Feb-2025
Web References: Nature Chemistry DOI
References: Daniel Mark Shapiro, et al., (2025) Synthetic biomolecular condensates enhance translation from a target mRNA in living cells, Nature Chemistry
Image Credits: Daniel Shapiro
Keywords: Synthetic biology, biological condensates, protein production, elastin-like polypeptides, mRNA, biopharmaceuticals, biotechnology, E. coli, condensates engineering, microbial synthesis, RNA concentration, therapeutic production.
Tags: antibiotics production in bacteriabacterial protein synthesis techniquesbiological condensates in cellsbiotechnology advancements for pharmaceuticalsDuke University biomedical engineeringefficient microbial production systemsindustrial applications of biotechnologymicrobial hosts optimizationmRNA trapping for protein synthesisprotein production enhancementsustainable protein manufacturingsynthetic biology innovations