A new technology developed by the “Bioengineering in Reproductive Health” team at the Institute for Bioengineering of Catalonia (IBEC) is able to visualise the metabolism of embryos obtained through in vitro fertilisation in order to decide which are most likely to implant correctly in the uterus and reaching full-term. It is a more accurate and reliable technique than traditional methods.
Credit: Institute for Bioengineering of Catalonia (IBEC)
A new technology developed by the “Bioengineering in Reproductive Health” team at the Institute for Bioengineering of Catalonia (IBEC) is able to visualise the metabolism of embryos obtained through in vitro fertilisation in order to decide which are most likely to implant correctly in the uterus and reaching full-term. It is a more accurate and reliable technique than traditional methods.
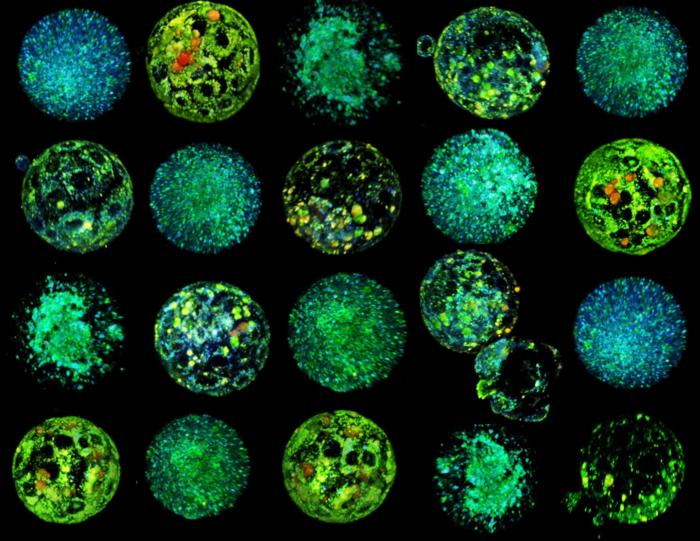
The revolutionary method, called METAPHOR”, generates 3D images that reveal the colours present in the embryo in a completely non-invasive way. Certain naturally fluorescent compounds in the embryo’s metabolism are also key to processes such as cellular respiration or nutrient consumption, making METAPHOR a reliable way to monitor the embryo’s health.
“This new technology will help to increase the probability of success in assisted reproduction processes, reducing the so-called ‘time to pregnancy’ and the economic and psychological burden on patients,” says Samuel Ojosnegros, principal investigator at IBEC and leader of the study.
The paper, published in the prestigious journal PNAS, describes how, in studies with mice, they were able to double the success rate in selecting viable embryos compared to embryologists using traditional microscopy. In addition to embryo analysis, the method is highly accurate in analysing oocyte metabolism, allowing the most suitable oocytes to be selected for in vitro fertilisation. To do this, they compared oocytes from young and older females, as age is known to be crucial for oocyte viability. The METAPHOR system discriminated between young and non-young oocytes with 96% accuracy and was able to predict which would develop into viable embryos with over 80% accuracy, an unprecedented figure in the field.
“We are able to assess the loss of egg quality associated with the loss of fertility with age. We look for so-called ‘molecular signatures’, characteristics of the cells that are associated with this loss of fertility, such as the distribution of mitochondria. From this information, we can predict which oocytes will develop and which will not. This would be a breakthrough in the management of fertility donation and preservation,” says Anna Seriola, senior researcher in the Ojosnegros group and author of the study.
The technological basis of METAPHOR uses artificial intelligence methods to analyse metabolic images obtained by hyperspectral microscopy. “Using hyperspectral microscopy, we acquire hundreds of images containing complex information of many mixed metabolites from embryos and oocytes. To analyse them, we trained an artificial intelligence tool capable of analysing and classifying these images in a few minutes,” says Albert Parra, a researcher in the Ojosnegros group and first author of the study.
The power and safety of the new method support METAPHOR as a revolutionary tool for assessing oocytes and embryos based on their physiology. Researchers are currently fine-tuning the technology to evaluate human embryos and have established a spin-off company to bring the technology to assisted reproduction clinics in the coming years.
Journal
Proceedings of the National Academy of Sciences
Method of Research
Experimental study
Subject of Research
Cells
Article Title
METAPHOR: Metabolic Evaluation through Phasor-based Hyperspectral Imaging and Object Recognition for Mammalian Blastocysts and Oocytes
COI Statement
The lead laboratory in this work has received private funding from the VC Scranton Enterprises. The authors declare that this funder has no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript.



