A novel vaccine strategy utilizing a self-destructing strain of Mycobacterium bovis Bacillus Calmette-Guérin (BCG) has shown promising results in combating tuberculosis (TB) among macaque monkeys, according to a groundbreaking study conducted by researchers at the University of Pittsburgh. Published in the esteemed journal Nature Microbiology on January 10, 2025, this research uncovers the enhanced safety and protective efficacy provided by an intravenous vaccination route, coupled with a unique bio-engineered mechanism to ensure the vaccine’s controlled biodegradation post-administration.
Historically, BCG vaccine injections have been the standard preventive measure against TB in humans, although they largely fail to confer adequate immunity in adults. The existing methodology of delivering the BCG vaccine, which relies on intradermal or subcutaneous administration, often leads to partial protection and an elevated risk of adverse immune reactions. This novel approach circumvents such limitations by introducing a strain designed to self-destruct, which could dramatically diminish the chances of vaccine-derived infections, particularly in individuals with weakened immune systems.
Dr. JoAnne Flynn, the leading scientist behind this research, emphasizes the implications of this innovative strategy for public health, especially in the face of the World Health Organization’s 2024 designation of TB as the deadliest disease worldwide. In her words, the inherent safety mechanisms integrated into the Kill-Switch BCG strain ensure that even immunocompromised patients can be vaccinated against TB without the lingering threat of an active mycobacterial infection. This insight into the dual action of the vaccine holds the potential to revolutionize how vaccinations are approached in vulnerable populations.
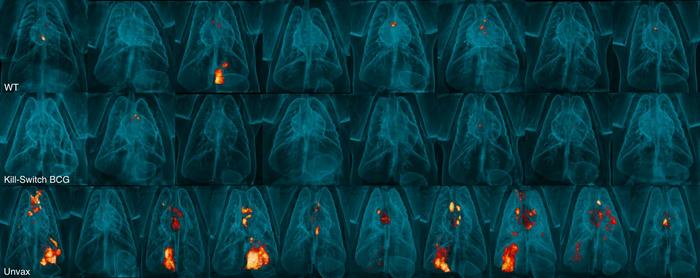
In the study, unvaccinated macaque monkeys displayed severe lung damage after being infected with Mycobacterium tuberculosis, indicative of the destructive potential of this pathogen. In stark contrast, those who received the self-destructing BCG vaccine demonstrated not only competent immune responses but also minimal signs of inflammation or disease post-infection. The middle row of their reported images aptly highlights that the Kill-Switch BCG vaccine was as effective, if not more so, than the conventional BCG method in eliciting robust immune protection.
Flynn’s esteemed team, collaborating with colleagues from Cornell University and the National Institutes of Health, previously established that intravenous administration of a standard BCG vaccine resulted in a remarkable bacterial load reduction within the lungs of macaques. The new study builds upon these findings by integrating an engineered safety switch that triggers the vaccine’s biodegradation upon the presence of doxycycline, an antibiotic, or upon cessation of its treatment. The mouse models utilized in the experiments displayed comparable immunity to standard BCG injections, alongside rapid elimination of the BCG strain from their systems, highlighting the potential for swift and safe vaccination protocols.
The enhanced immune response observed in macaque monkeys vaccinated with the newly developed BCG strain is promising. Eight weeks following an exposure to live Mycobacterium tuberculosis, none of the monkeys showed any detectable levels of lung inflammation, and six out of eight were devoid of recoverable live bacteria, showcasing the superior efficacy of the Kill-Switch BCG over traditional methods. Such results underscore a critical evolution in the landscape of TB vaccination strategies, a field that has seen limited advancements for decades.
The implications of this study extend beyond mere animal research; they enter the realm of potential human application. With the easy-to-engineer components that define the Kill-Switch BCG, researchers aim to alleviate longstanding safety concerns associated with the intravenous administration of live vaccines. As this avenue of research progresses towards human clinical trials, anticipation builds regarding its impact on public health policy and TB management.
Despite the challenges faced in bringing this vaccine to clinical testing, the optimism shared by Flynn and her co-authors cannot be overstated. They believe that with rigorous investigation, this intravenous vaccination approach could become a staple in preventive healthcare, especially for those most at risk of contracting TB due to compromised immune systems. As the world grapples with this persistent infectious disease, innovative solutions remain desperately needed.
This pursuit to harness a self-sufficient mechanism within vaccines represents a technological achievement in biotechnology and immunology. As the existing BCG retains a role in combating childhood TB effectively, the new findings suggest that the advent of self-eliminating vaccines could parallel public health advancements in preventing more lethal outcomes associated with Mycobacterium tuberculosis.
In conclusion, the research carried out by the University of Pittsburgh marks a pivotal moment in TB vaccine development. By demonstrating the efficacy of the self-destructing BCG strain and its potential for safe intravenous administration, the findings contribute to a growing body of evidence that suggests a sustainable and effective future in vaccine technology. As society looks towards more resilient public health strategies, the implications of this study highlight the need for an evolved perspective on vaccination in the fight against tuberculosis—a relentless adversary.
Subject of Research: Self-destructing BCG vaccine for tuberculosis
Article Title: A BCG kill switch strain protects against Mycobacterium tuberculosis in mice and non-human primates with improved safety and immunogenicity
News Publication Date: 10-Jan-2025
Web References: Nature Microbiology
References: DOI: 10.1038/s41564-024-01895-4
Image Credits: Pauline Maiello
Keywords: Tuberculosis, Vaccination, Animal research, Bacterial infections, Mycobacteria, Nonhuman primates, Public health, Vaccine development, Intravenous injections, Immunity