Credit: @Science China Press
There is an urgent need for high-energy-density rechargeable batteries to further satisfy the ever-growing demand for electrical energy storage devices. Triggering the O-related anionic redox activities (e.g. typical Li/Na/K-O2 battery, and Li/Na-rich cathodes) have been regarded as the most promising capacity-boosting strategy for batteries. However, the practical realization of Li/Na/K-O2 battery, a gas-open cell architecture, is severely plagued by some gaseous O2-related intrinsic defects. For example, porous air cathode is easily clogged by hosting the solid O2 reduction products, resulting in the practical stored energy reveals far below the theoretical value. Moreover, due to the phase changes between gaseous O2 and solid Li/Na/KxO, sluggish kinetic obstacle leads to large round-trip overpotential. Besides, air purifier devices or O2 storage cylinder have to be equipped, and further drag the energy density promotion.
In a new research published in the Beijing-based National Science Review, scientists at the Nanjing University in China, and at National Institute of Advanced Industrial Science and Technology in Japan present the realization of a reversible superoxide-peroxide conversion in a K-based high-capacity rechargeable sealed battery device.
Co-authors Yu Qiao and Haoshen Zhou likewise outline the potential development directions and design principle of this reversible superoxide/peroxide (KO2/K2O2) inter-conversion on KO¬2-based cathode system for potassium-ion battery (KIB) technology.
“Traditionally, A novel synergistical modification ideal is abandoning the utilization of gaseous O2, and controlling the high-energy-density oxygen-based redox reaction processes within the redox interconversion among different solid phases (a more commercialized sealed cell environment).” they state in an article titled “A high-capacity cathode for rechargeable K-metal battery based on reversible superoxide-peroxide conversion”
“In 2019, our group successfully trapped the O-related redox activity within a reversible peroxide-oxide (Li2O2/Li2O) interconversion stage (Nature Catalysis, 2019, doi.org/10.1038/s41929-019-0362-z), and achieved a novel high-energy-density Li-ion battery. However, to the best of our knowledge, the interconversion between superoxide-peroxide has not been reported yet, which is another potential high-energy-density redox candidate.” Qiao and Zhou state. They also point out that “The most difficult point to realize the reversible superoxide-peroxide interconversion focused on the stabilization of superoxide.”
“In this study, we originally achieved the reversible interconversion between superoxide (KO2) and peroxide (K2O2) in a rechargeable battery system, and made great improvements on the specific capacity for potassium-ion (K-ion) battery.” they state.
They point out that there are two key issues achieved in this work:
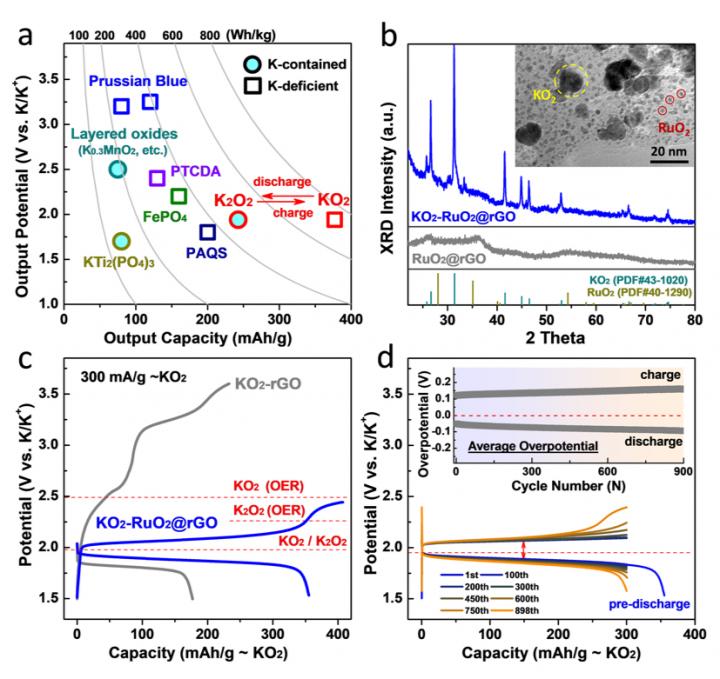
1) On the aspect of “Chemistry”: For the first time, the reversible superoxide-peroxide interconversion has been realized, and they successfully restrained the irreversible oxygen loss (O2 evolution, etc.). By the employment of advanced systematical operando spectroscopies (in-situ Raman + SERS + GC-MS), the authors originally found the formation of nucleophilic superoxo anion (O2-) was the chief criminal for the irreversible redox behavior. Moreover, by the sharp comparison verse blank group, they proved the hybridization between RuO2 catalyst and K-deficient K1-xO2 can induce the formation of stable surface protection layer (proved by hard-XAS spectroscopy) and prevent the oxygen loss. For the first time, the essential difference between moderate superoxide and dangerous superoxo anion (O2-) has been clarified. “We believe these findings would be full of novelty on the viewpoint of “Chemisty”.” they state.
2) On the aspect of “Battery”: For the practical battery technology, the development of K-ion battery is severely hindered by the limited capacity of cathode candidates (typically around 100 mAh/g cathode capacity). In this work, benefitting from the high-capacity KO2/K2O2 redox couple, the cathode capacity has been largely boosted to 300 mAh/g. This sealed battery system is totally different from previous reported gas-open K-O2 battery (O2/KO2 conversion). Moreover, the round-trip overpotential has been successfully restrained within 0.2 V (at quite high current rate of 300 mA/g), indicating a high energy efficiency around 90%. The reversible cycling can be achieved around 900 times, indicating remarkable long-term cycling stability. Besides, not only for half-cell mode, after electrolyte modification, a practical full-cell has been fabricated with high-energy-density and superior cycle stability. “We believe these large improvements present great significance on the practical “Battery” level.” Qiao and Zhou state.
“We believe that the demonstration of a superoxide/peroxide redox dominated battery system with ultralong cycle stability will open up a new gate and spur the development of more effective catalytic cathode frameworks.” Qiao and Zhou state. “More importantly, the identification of the feasibility (from the mechanism/chemistry perspective) and the realization of the impressive cyclability (from the practical viewpoint) herein would stimulate the development of oxygen-based anionic redox activity in enhancing the energy density of rechargeable battery technologies.” they added. “The desirable features revealed in the current battery system also triggers a design direction for synergistically combining the electrode and electrocatalyst materials, engineering the next-generation high-energy-density battery technologies.”
###
This research received funding from the National Basic Research Program of China and the National Natural Science Foundation of China.
See the article:
Yu Qiao, Han Deng, Zhi Chang, Xin Cao, Huijun Yang, and Haoshen Zhou
A high-capacity cathode for rechargeable K-metal battery based on reversible
superoxide-peroxide conversion
Natl Sci Rev (Nov.27 2020)
https:/
Media Contact
Haoshen Zhou
[email protected]
Original Source
http://doi.
Related Journal Article
http://dx.




