G protein-coupled receptors (GPCRs) are the largest and most diverse group of cell surface proteins in humans. These receptors, which can be seen as ‘traffic directors,’ transmit signals from the outside to the inside of cells and are involved in many physiological processes. Given their prominent roles in cellular communication, cell growth, immune responses, and sensory perception, many drugs have been developed to target GPCRs, for the treatment of conditions such as asthma, allergies, depression, hypertension, and heart disease. In fact, more than 300 GPCR-related drugs are currently in clinical trials, 36% of which target over 60 novel GPCR targets without an already-approved drug. Moreover, drugs that target GPCRs account for as much as 27% of the global market share of therapeutic drugs, with aggregated sales close to US$890 billion between 2011 and 2015. Thus, any technique that could accelerate research on GPCRs is likely to trigger a large ripple effect, ultimately bringing more effective treatments to millions of people.
Credit: Mitsunori Shiroishi from Tokyo University of Science
G protein-coupled receptors (GPCRs) are the largest and most diverse group of cell surface proteins in humans. These receptors, which can be seen as ‘traffic directors,’ transmit signals from the outside to the inside of cells and are involved in many physiological processes. Given their prominent roles in cellular communication, cell growth, immune responses, and sensory perception, many drugs have been developed to target GPCRs, for the treatment of conditions such as asthma, allergies, depression, hypertension, and heart disease. In fact, more than 300 GPCR-related drugs are currently in clinical trials, 36% of which target over 60 novel GPCR targets without an already-approved drug. Moreover, drugs that target GPCRs account for as much as 27% of the global market share of therapeutic drugs, with aggregated sales close to US$890 billion between 2011 and 2015. Thus, any technique that could accelerate research on GPCRs is likely to trigger a large ripple effect, ultimately bringing more effective treatments to millions of people.
Today, approaches such as cryo-electron microscopy, optogenetics, computational approaches and artificial intelligence, biosensors and label-free technologies, and single-cell technologies are being explored for GPCR drug discovery and development. Among them, the single-cell approach based on yeast is one of the most useful platforms to study GPCRs. Besides its widespread application in beer and bread making, the yeast species Saccharomyces cerevisiae has a long history of being used as a host to research human derived GPCRs. Although some GPCRs can be engineered to enhance their stability and function to facilitate experiments, most GPCRs do not function well in yeast cells. This long-standing problem has greatly slowed progress in our understanding of GPCRs and the development of new drugs that target them.
Against this backdrop, a research team from Tokyo University of Science (TUS), Japan, recently came up with an innovative strategy to restore the activity of human derived GPCR human histamine 3 (H3R) in S. cerevisiae. Their study, published in Volume 13 of Scientific Reports on September 26, 2023, was led by Associate Professor Mitsunori Shiroishi and co-authored by Ms. Ayami Watanabe and Ms. Ami Nakajima, all from TUS.
“H3R is mainly expressed in the nervous system. It is involved in cognitive function, and its inhibition is associated with the therapeutic outcomes of various conditions, such as ADHD, schizophrenia, Alzheimer’s disease, and narcolepsy,” explains Dr. Shiroishi. Through preliminary experiments, the team showed that H3R becomes non-functional when expressed in yeast.
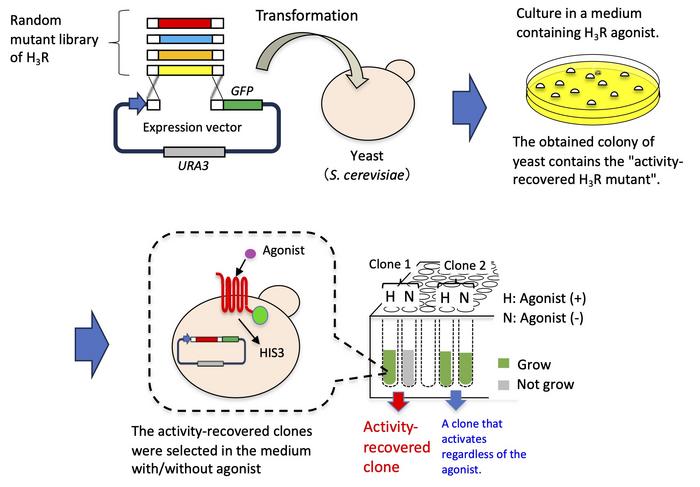
To restore its function, the research team utilized a technique called error-prone polymerase chain reaction to introduce random mutations in the H3R gene. After producing a random mutant library of H3R, they introduced modified DNA segments into yeast cells and cultivated them in the presence of an H3R agonist—a compound that binds to H3R and sets off a measurable response. By screening through multiple cultures, the researchers obtained four mutants in which the normal activity of H3R was restored. These mutants responded exclusively to a type of yeast strain that harbors certain G-chimera proteins. The mutations responsible for the restored activity were located near the amino acid sequence motifs important for GPCR activation.
This innovative approach to study GPCRs could have profound implications, particularly in the fields of medicine and cell biology. “Our research could help elucidate the function of GPCRs and may even lead to the development of drugs with fewer side effects, as well as bolster drug discovery for diseases for which there is currently no treatment,” remarks Dr. Shiroishi. There are many therapeutic areas where GPCR-targeting drugs are being actively developed, including neurological disorders like Alzheimer’s and schizophrenia, cardiovascular diseases such as hypertension and heart failure, various types of cancer, and metabolic disorders.
A deeper understanding of GPCR variations and how they impact individuals differently could also lead to new approaches to personalized medicine. Tailoring GPCR-targeted drugs to an individual’s genetic makeup and their specific disease profile may greatly improve treatment outcomes. Furthermore, generic GPCR treatments reaching a vast number of people worldwide might also become a reality, which would reduce the burden on healthcare systems.
We are certain that the findings of this study will pave the way to a healthier future for everyone.
***
Reference
DOI: https://doi.org/10.1038/s41598-023-43389-z
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society,” TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Associate Professor Mitsunori Shiroishi from Tokyo University of Science
Dr. Mitsunori Shiroishi graduated from the Department of Biomolecular Engineering at Tohoku University, Japan, in 1998 and completed his doctoral degree there in 2003. He joined Tokyo University of Science as an Associate Professor in April 2018. He currently leads the Protein Engineering Laboratory as Principal Investigator, where his team conducts cutting-edge research on protein science focused mainly on cell receptors with pharmaceutical potential, including receptors with immunoglobin-like fold and G protein-coupled receptors. He has published over 50 research papers, which have received more than 3,500 citations.
Journal
Scientific Reports
DOI
10.1038/s41598-023-43389-z
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Recovery of the histamine H3 receptor activity lost in yeast cells through error-prone PCR and in vivo selection
Article Publication Date
26-Sep-2023
COI Statement
The authors declare no competing interests




