The new REsolution consortium is a public-private research partnership, supported by the Innovative Medicines Initiative (IMI), with nine partners from academia and the pharmaceutical industry. Starting on June 1, 2021 and with a duration of 2 years, the project aims to understand how genetic variants in humans affect the function of hundreds of cellular transporters.
How do molecules such as vitamins, nutrients and drugs enter our organs and cells? Why do some of us take up certain molecules more easily than others? The REsolution consortium studies how differences in the genetic makeup of so-called transporter genes, encoding proteins that allow molecules to pass cellular membranes, may account for those differences. The REsolution consortium includes universities, research institutes, a small-medium-sized enterprise, and European Federation of Pharmaceutical Industries and Associations (EFPIA) members. The project, led by Pfizer and the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, has received funding through the IMI joint undertaking consisting of €1 million from the H2020 Programme of the European Union and €1 million from in-kind contributions of industry partners.
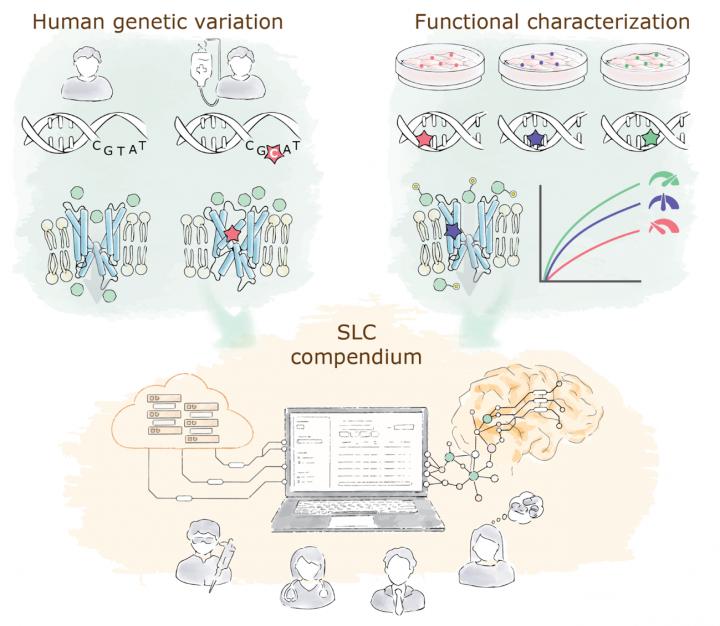
Solute carriers (SLCs) are transport proteins located on cellular membranes and can be seen as cellular “gates”. With more than 400 proteins, they constitute the largest family of transporters in the human genome and represent a largely untapped source of potential novel drug targets. They are essential for a cell’s well-being and often genetically associated with human diseases – such as amyotrophic lateral sclerosis (ALS), autism, Alzheimer’s disease, schizophrenia, diabetes, metabolic and cardiovascular diseases, and several types of cancer – and play an integral role in drug absorption into specific organs.
“In the last decade, the amount of data on human genetic variations has skyrocketed,” says the academic coordinator Giulio Superti-Furga from CeMM. “The REsolution initiative offers the chance of interpreting what these variations mean in terms of transporter functions and our individual ability to access molecules from the environment. It should create a large impact on medical and, particularly, pharmacological research.”
REsolution aims at gathering publicly available datasets and combining them with novel experimental data on genetic variants of SLCs. To that end, REsolution will build on the ongoing project RESOLUTE, which is working efficiently on a systematic campaign to increase the knowledge on SLCs by creating tools and datasets and making them available to the scientific community.
“REsolution is the natural evolution of our collective work to advance a more holistic view of the SLC protein family,” said Claire Steppan, the EFPIA project lead from Pfizer. “Through the development of this rich dataset, we hope to enhance the broader scientific community’s understanding of SLCs and ultimately accelerate the development of promising therapeutics for patients in need.”
RESOLUTE and REsolution together have the potential to create an unprecedented SLC database. This resource could act as a “compass” to allow the medical community and drug discoverers to prioritize those targets most clearly involved in specific human diseases.
###
For more information visit: https:/
The REsolution project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 101034439. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
The IMI is a partnership between the European Union and the European pharmaceutical industry. Since 2008, IMI has facilitated open collaboration in research to advance the development of, and accelerate patient access to, personalised medicines for the health and wellbeing of all, especially in areas of unmet medical need. https:/
This communication reflects only the authors’ views and neither IMI nor the European Union and EFPIA are responsible for any use that may be made of the information contained therein.
Media Contact
Anna Maria Schwendinger
[email protected]