Recently, a research team led by Dr. ZHU Yingjie from the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences has published a study in Neuron, the study presents a comprehensive transcriptional profile of the lateral septum (LS) at the single-cell level, elucidating the spatial distribution of its major neuronal types. The study shows that neurons expressing estrogen receptor 1 (LSEsr1), predominantly located in the ventral subregion of LS, play a crucial role in reward-seeking and methamphetamine (METH) addiction.
Credit: ZHU Yingjie
Recently, a research team led by Dr. ZHU Yingjie from the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences has published a study in Neuron, the study presents a comprehensive transcriptional profile of the lateral septum (LS) at the single-cell level, elucidating the spatial distribution of its major neuronal types. The study shows that neurons expressing estrogen receptor 1 (LSEsr1), predominantly located in the ventral subregion of LS, play a crucial role in reward-seeking and methamphetamine (METH) addiction.
In 1954, psychologists Olds and Milner discovered the brain’s reward system through intracranial self-stimulation (ICSS) experiments in rats, which inspired a large body of research on the neural basis of reward, motivation and learning. By implanting electrodes in the rat brain, they found that rats would persistently press a lever to receive electrical stimulation in certain brain regions, even neglecting basic needs like eating and drinking.
The septal area was identified as one of the regions with the strongest effects in supporting self-stimulation, though the neural types that mediating the rewarding effects of septal electrical stimulation remained elusive until the current study.
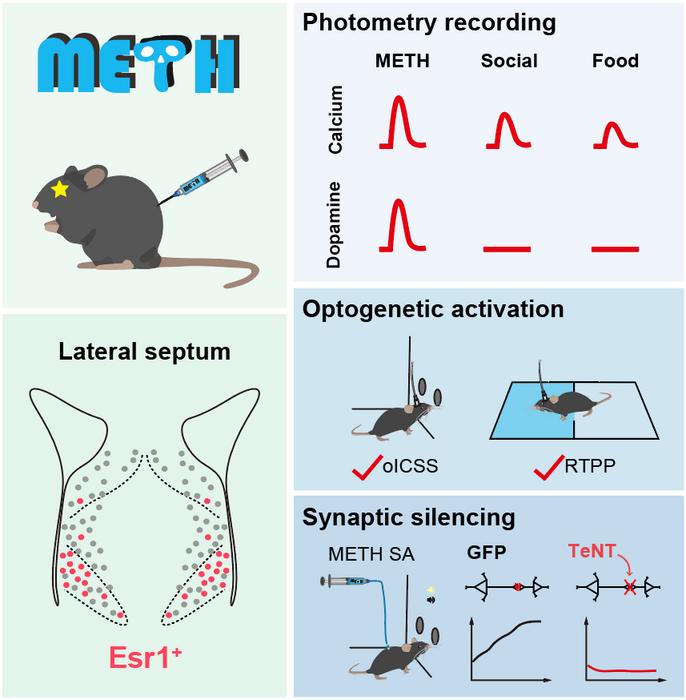
Using single-nucleus RNA-Seq and novel spatial genomics technology (MiP-seq), Dr. ZHU’s team mapped the transcriptional profile of the septal area, identifying its major neuronal types and their spatial distribution. Optogenetic activation of different neuronal types in the LS revealed that LSEsr1 neurons promote dopamine release by disinhibiting dopamine neurons in the ventral tegmental area (VTA), though which LSEsr1 neurons drives reward-seeking behavior.
The VTA-NAc mesolimbic pathway is the core component of brain’s reward system, where both natural rewards (palatable food, social interactions) and addictive drugs (METH, opioids) increase dopamine levels in the NAc.
Intriguingly, the team found that the LS also receives dopaminergic projections from the VTA. METH, but not palatable food or social reward, elevates dopamine concentration in the LS, suggesting that dopamine release in the LS might be a characteristic property of addictive substances. Correspondingly, silencing of LSEsr1 neurons abolishes METH reward without affecting natural rewards, highlighting a novel cellular and circuit target for specific intervention of drug addiction.
After repetitive METH exposure, the excitability of LSEsr1 neurons were enhanced due to an upregulation of HCN1 cation channels. Specific knockdown of the HCN1 channels in LSEsr1 neurons allows mice to still experience METH reward but eliminates behavioral sensitization.
This finding suggests that the HCN1 channel is crucial in the development of sensitization and addiction to METH. Considering that manipulation of LSEsr1 neurons does not affect natural rewards, targeting these neurons and the HCN1 channels could be a promising strategy for treating drug addiction.
Journal
Neuron
DOI
10.1016/j.neuron.2024.06.004
Method of Research
Commentary/editorial
Subject of Research
Animals
Article Title
Cellular and circuit architecture of the lateral septum for reward processing
Article Publication Date
2-Jul-2024




