A groundbreaking advancement in the realm of green hydrogen production has been achieved by a research team led by Professor YAN Ya from the Shanghai Institute of Ceramics of the Chinese Academy of Sciences. This collaboration, which spans institutions including Huazhong University of Science and Technology, Shanghai Jiao Tong University, and the University of Auckland, has resulted in the development of a highly stable and incredibly efficient water oxidation catalyst. The team’s discovery marks a decisive leap forward, reshaping the landscape of water splitting technology that underpins sustainable hydrogen generation.
Published in the journal Science on April 25, 2025, their study addresses one of the most challenging hurdles in electrolytic water splitting: water oxidation. This half-reaction, in which water molecules are split into oxygen gas, protons, and electrons, demands high energy input due to its sluggish kinetics and complex multi-electron transfer processes. The inefficiency of water oxidation curtails the overall productivity of hydrogen generation systems, necessitating catalysts that can operate stably and efficiently under harsh industrial conditions.
Traditional transition metal-based catalysts have shown promise in facilitating the water oxidation reaction, especially under alkaline conditions. Nevertheless, their performance often deteriorates rapidly when subjected to industrial-relevant high current densities. Structural distortions within the catalyst and the dissolution of catalytically active metal sites during oxidative stress cause significant degradation. This instability restricts the catalyst’s practical application in large-scale hydrogen production, where both activity and durability are non-negotiable.
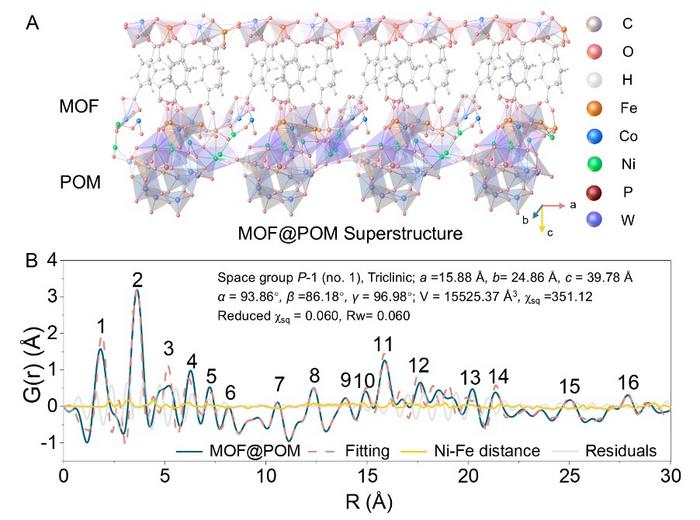
To surmount these challenges, the researchers devised a novel superstructure catalyst by strategically grafting cobalt-iron (CoFe) metal-organic frameworks (MOFs) onto nickel-bridged polyoxometalates (POMs). This unique integration creates a hierarchical MOF@POM architecture, wherein the CoFe-MOF transforms in situ under oxidation conditions into an ultrathin single-layer CoFe layered double hydroxide (CoFe-LDH). Crucially, this hydroxide layer is covalently bonded to the POM units through robust Ni–O bridges, resulting in a composite catalyst that blends exceptional catalytic activity with remarkable structural resilience.
In situ electrochemical spectroscopic techniques provided crucial insights into the working mechanism of this catalyst. The interplay between the cobalt and iron active sites and the nickel and tungsten elements acting as tuning centers generates a synergistic catalytic process. As the catalyst operates, the oxidation states of cobalt and iron increase, indicative of their active participation in oxygen evolution. Simultaneously, Ni–O and W–O components undergo dynamic valence oscillations, which serve to modulate the electron density within the catalyst, enhancing its responsiveness and stability during prolonged electrolysis.
Electrochemical testing revealed that the CoFe-LDH@POM catalyst achieves a remarkably low overpotential of only 178 millivolts at a current density of 10 milliamperes per square centimeter in alkaline electrolytes. This performance surpasses many conventional transition metal-based water oxidation catalysts, setting a new standard for energy-efficient oxygen evolution reactions. Furthermore, when incorporated into an anion exchange membrane electrolyzer, the catalyst enables operation at an industrial-scale current density of 3 amperes per square centimeter with a cell voltage of merely 1.78 volts at 80 degrees Celsius, exceeding the rigorous targets set forth by the U.S. Department of Energy for 2025.
Longevity tests underscore the catalyst’s robustness, with the electrolyzer demonstrating stable operation over 5,140 hours at 2 amperes per square centimeter under ambient temperature conditions. Importantly, the system exhibits an extremely low voltage decay rate of just 0.02 millivolts per hour, indicative of minimal degradation. Even at an elevated temperature of 60 degrees Celsius, the device maintained continuous operation for more than 2,000 hours, signaling its potential for real-world industrial deployment where thermal and operational stability are integral.
This breakthrough not only delivers an extraordinary water oxidation catalyst but also establishes a comprehensive design framework for future electrocatalysts. By harnessing the sophisticated interplay between layered metal hydroxides and polyoxometalate units, it opens pathways for constructing catalysts that combine high activity and exceptional durability. Such advancements pave the way toward scalable, low-energy alkaline water electrolysis systems, which are essential for meeting growing global hydrogen demands sustainably.
The researchers’ approach exemplifies how multifaceted strategies—integrating material chemistry, in-situ spectroscopic investigations, and electrochemical engineering—can converge to overcome long-standing challenges. The MOF@POM superstructure catalyst, with its finely-tuned electronic and mechanical properties, demonstrates how deliberate molecular architecture design can revolutionize catalytic processes vital for the clean energy transition.
As the hydrogen economy accelerates worldwide, innovations of this caliber will be key in bridging the gap between laboratory breakthroughs and industrial application. The enduring stability and exceptional efficiency of the CoFe-LDH@POM catalyst present a promising avenue to power future electrolyzers capable of reliable, high-throughput hydrogen production with minimal energy input, advancing the realization of a carbon-neutral energy future.
Subject of Research: Water Oxidation Catalyst for Green Hydrogen Production
Article Title: Polyoxometalated metal-organic framework superstructure for stable water oxidation
News Publication Date: 25-Apr-2025
Web References: DOI: 10.1126/science.ads1466
Image Credits: YAN Ya
Keywords
Water oxidation, Catalysis, Industrial production, Electron density, Kinetic stability, Alkalinity, Hydrogen production, Water electrolysis, Molecular targets, Metal organic frameworks
Tags: efficient hydrogen generation systemselectrolytic water splitting technologygreen hydrogen productionhigh current density performanceindustrial conditions for catalysismulti-electron transfer processesProfessor YAN Ya researchShanghai Institute of Ceramicsstable water oxidation catalystsustainable energy advancementstransition metal-based catalystswater oxidation catalysis