Credit: Graphic by Julie McMahon
CHAMPAIGN, Ill. — Its name is Stygobromus hayi, the Hay's Spring amphipod. It is spineless. It lacks vision. It is an opportunistic feeder, consuming whatever resources are available – perhaps including the remains of its own kind.
That is where its similarities to some of Washington, D.C.'s more notorious megafauna end. And while this tiny creature has been the subject of scientific inquiry in recent years, researchers report on a way to survey it without threatening its existence, as other studies had done.
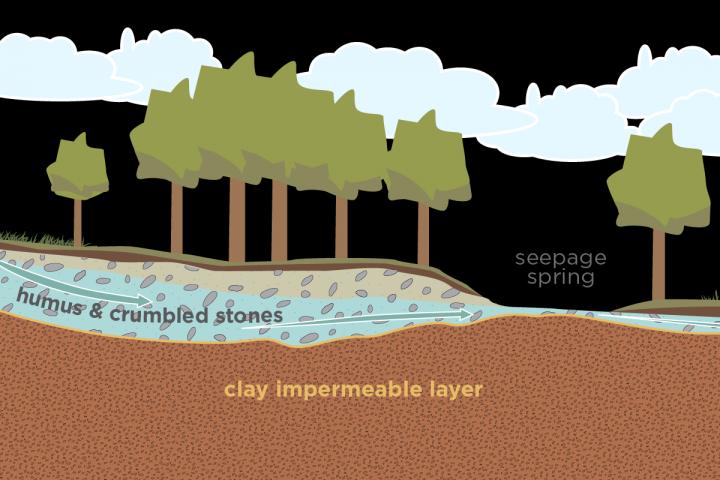
The Hay's Spring amphipod lives in swampy areas called seepage springs where groundwater sometimes spills out onto the surface of Rock Creek Park, in Washington, D.C., its only known home in the world. Because it is so rare, and perhaps also because of its prestigious ZIP code and proximity to the National Zoo, this tiny crustacean is on the U.S. endangered species list.
"Yes, it's small, it's white, it's eyeless, it lives underground," said Matthew Niemiller, an ecologist with the Illinois Natural History Survey who led a recent study to search for the creature in its Rock Creek Park home. "It's not a cute, cuddly or charismatic species. But we're still learning more and more about groundwater ecosystems. And there is evidence that these crustaceans are important indicators of groundwater quality, and may play important roles in water purification and nutrient cycling over time."
The Hay's Spring amphipod's subterranean habitat makes it particularly difficult to study, Niemiller said.
"To find out where species are located and how many there are, we have to disturb the site," he said.
This normally involves sifting through waterlogged leaves in muddy seepage areas, where underground springs leak out to the surface.
"And because these critters are small – no more than a centimeter long – and a couple of other species look very similar to S. hayi, we would have to sacrifice any individuals we find and take them back to the lab to identify them," he said. "Since this is an endangered species, that's something we don't want to do."
Niemiller and his colleagues decided to try a different approach, called eDNA. They sampled water in seepage springs where S. hayi and other Stygobromus species had previously been sighted and in others where they had not. The team filtered the water on site, returning to the lab to check their filters for the animal's DNA.
"This environmental DNA approach has successfully detected larger aquatic animals like salamanders, but no studies had previously used this technique to hunt for invertebrates in groundwater," Niemiller said.
All creatures shed their DNA into the environment, but the researchers didn't know if their target animal would slough off enough genetic material to be detectable in their samples. Water quality also was an issue: The seepage springs are muddy and loaded with acidic plant tannins, which can inhibit polymerase chain reaction, the key method for amplifying and detecting DNA in environmental samples.
The researchers overcame the latter problem, however, using chemicals known to remove potential PCR inhibitors.
Their analysis detected S. hayi DNA in samples from three sites where the creature had been seen before, and in a fourth new seepage spring nearby. The researchers also found the DNA of a closely related species, Stygobromus t. potomacus, in four of 10 springs sampled.
"We now know that eDNA is going to be a really worthwhile tool – at least for aquatic species in difficult-to-access habitats," Niemiller said. "You could go to a spring outflow, take a water sample, and see what you might detect there with a limited outlay of labor, time and money."
The method has its limitations, however. Environmental DNA sampling can tell researchers whether something is present or absent, but not how many of the targeted organisms are present.
"We also don't know how long DNA persists in these systems and whether we're detecting animals that are still alive," Niemiller said.
The new study demonstrates, however, that this is a viable method to supplement other, more detailed studies of aquatic organisms, he said.
"As funding becomes tighter, we need to think about more efficient ways to monitor and protect these species," he said.
###
The study is reported in the journal Conservation Genetics Resources. The research team also included biologists David Culver and Daniel W. Fong and students Jenna Keany and Heather Gilbert, from American University; Megan Porter, from the University of Hawaii; Christopher Hobson, from the Virginia Department of Conservation and Recreation; K. Denise Kendall, from the University of Illinois; and Mark A. Davis and Steven J. Taylor, from the INHS.
The INHS is a division of the Prairie Research Institute at the University of Illinois at Urbana-Champaign.
Editor's notes:
To reach Matthew Niemiller, call 217-244-1122; email [email protected].
The paper "Evaluation of eDNA for groundwater invertebrate detection and monitoring: A case study with endangered Stygobromus (Amphipoda: Crangonyctidae)" is available online and from the U. of I. News Bureau.
DOI: 10.1007/s12686-017-0785-2
Media Contact
Diana Yates
[email protected]
217-333-5802
@NewsAtIllinois
http://www.illinois.edu
Related Journal Article
http://dx.doi.org/10.1007/s12686-017-0785-2
############
Story Source: Materials provided by Scienmag