Credit: UT Southwestern
DALLAS – November 2, 2016 – Researchers have identified the first two core genes that regulate the amount of deep sleep and dreaming, a key development they believe will lead to the discovery of a network of related genes controlling sleep.
The study from the Peter O'Donnell Jr. Brain Institute demonstrates in mice that a single gene controls the amount of non-REM (rapid eye movement) sleep, which includes deep sleep. A second gene controls the amount or need for REM sleep, associated with vivid dreaming. The findings provide a critical molecular entry point to explain how sleep works and to identify potential targets to better treat sleep disorders.
"This research is just the beginning. We believe that these two genes are the first of many that regulate sleep," said study co-author Dr. Joseph S. Takahashi, Chairman of Neuroscience with the O'Donnell Brain Institute at UT Southwestern Medical Center and Investigator in the Howard Hughes Medical Institute.
Previous research has identified genes that regulate the switch between wakefulness and sleep. But until this latest study in Nature, scientists have not known what mechanisms control the drive or need for non-REM sleep, nor the amount of REM sleep.
Learn More
- How we sleep
- Study in Nature
- Takahashi Lab
- Blog: Get more sleep with good sleep hygiene
To find out, researchers used a forward-genetic approach in which they screened for sleep disorders in 8,000 mice using electroencephalogy (EEG) to monitor brain waves. They found two distinct pedigrees of note:
- Sleepy – A mouse they called Sleepyhad 50 percent more non-REM sleep than normal mice without any other obvious defects, caused by a mutation in the Salt-Inducible Kinase 3 Sik3 (Sik3) gene.
- Dreamless – A mouse researchers called Dreamlesswas severely deficient in the amount of REM sleep, a stage of rest characterized by rapid eye movements and vivid dreams. This deficit was caused by a mutation in the Sodium Leak Channel Non-selective (Nalcn) gene.
Researchers introduced these same mutations into normal mice and saw their sleep behaviors change accordingly.
"We hope this is the entry door to the black box that explains how our sleep is regulated," said the senior co-author Dr. Masashi Yanagisawa, an Adjunct Professor of Molecular Genetics at UT Southwestern and former HHMI Investigator. He now directs the International Institute for Integrative Sleep Medicine (IIIS) at the University of Tsukuba in Japan, where most of the mice were screened.
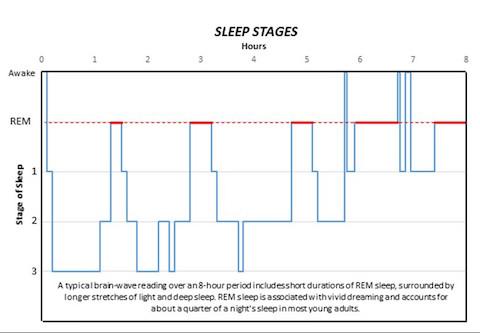
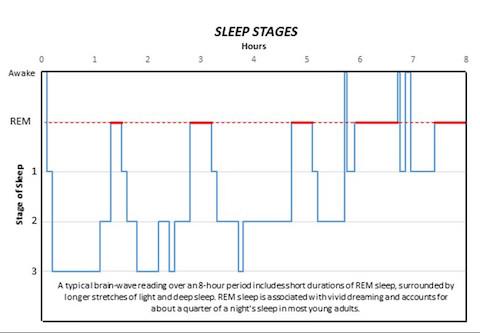
Normal sleep patterns include short durations of REM sleep surrounded by longer stretches of non-REM sleep and account for about a quarter of a night's rest in most young adults. Many forms of sleep disorder distort these patterns. Because the Sik3 and Nalcn genes have just been identified, no evidence yet exists to link them directly to known sleep disturbances in humans.
However, while the role and importance of REM sleep remains a point of debate, many scientists agree this stage of rest is involved in the formation of emotional memories and coping with negative experiences. Thus, a lack of REM sleep may contribute to conditions such as posttraumatic stress disorder (PTSD).
"At least in theory, this study opens up future possibilities to create new sleep-regulating drugs, but doing so will occur in the distant future," said Dr. Yanagisawa, noting that the proteins produced by Sik3 and Nalcn could possibly be molecular targets for new medicines.
Dr. Takahashi used a forward-genetic approach two decades ago to make a landmark discovery of the Clock gene that regulates the body's biological clock. The finding led his team to discover a network of more than 20 other related genes.
Dr. Takahashi said he expects the screen for sleep genes will lead to more genes, forming perhaps a much larger group than the clock genes because sleep affects more parts of the brain.
What's unclear is how big a part the other genes in that network play in regulating sleep. The Takahashi lab found that only a handful of the clock genes have a crucial role in the larger network.
"If the same is true for sleep, this is going to be a simplifying, illuminating discovery," said Dr. Takahashi, holder of the Loyd B. Sands Distinguished Chair in Neuroscience and 2016 recipient of the Peter Farrell Prize in Sleep Medicine.
Dr. Takahashi said he had wanted to conduct such a genetic screen for sleep mutants for many years but had to overcome logistical issues to conduct a large-scale effort. Most mouse studies involve no more than a few dozen animals, but Dr. Yanagisawa rapidly scaled up and optimized his lab's ability to screen large numbers of mice initially at UT Southwestern and now at his institute in Japan.
"To be able to screen 8,000 mice is something that most people would say is too much work," said Dr. Takahashi, explaining that each mouse had to be surgically wired for the EEG readings, among other steps. "Technically, this project was very challenging."
###
About UT Southwestern Medical Center
UT Southwestern, one of the premier academic medical centers in the nation, integrates pioneering biomedical research with exceptional clinical care and education. The institution's faculty includes many distinguished members, including six who have been awarded Nobel Prizes since 1985. The faculty of almost 2,800 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide medical care in about 80 specialties to more than 100,000 hospitalized patients and oversee approximately 2.2 million outpatient visits a year.
This news release is available on our website at http://www.utsouthwestern.edu/news.
To automatically receive news releases from UT Southwestern via email, subscribe at http://www.utsouthwestern.edu/receivenews
How We Sleep
A study from the Peter O'Donnell Jr. Brain Institute has identified the first two core genes that regulate the amount of deep sleep and dreaming. While normal sleep patterns include short durations of rapid eye movement (REM) sleep surrounded by longer stretches of non-REM sleep, the study published in Nature demonstrates how one gene can alter these patterns. Here's a look at the stages of sleep:
REM: Rapid eye movement sleep, associated with vivid dreaming. Brain waves are similar to those experienced during wakefulness. Breathing, heart rate and blood pressure increase. Muscles become paralyzed, protecting the person from acting out dreams. Person is more likely to wake from REM than non-REM sleep, though the awakenings usually last only a few seconds. While the role and importance of REM sleep remains a point of debate, many scientists agree it is involved in the formation of emotional memories and coping with negative experiences.
Stage 1: Between wakefulness and sleep. The heart rate begins to slow and breathing becomes regular. Dreaming is relatively rare. The person may be aware of sounds and may have quick body jerks. If awakened, the person will often believe they were not asleep. Brain waves begin to transition from beta/gamma to slower alpha waves, then theta waves.
Stage 2: Muscle activity decreases and awareness of outside sounds recedes. Sigma waves, or sleep spindles, provide short bursts of brain activity that combine with other low and high voltage peaks. These protect the person's sleeping state from outside disruptions and help with processing memory and other information. The person passes through this stage several times each night, usually accounting for about half the total sleep.
Stage 3: Deep sleep. The person is completely removed from outside stimuli and will feel groggy if awakened. Heart rate, breathing and blood pressure are at their lowest levels. Dreaming is more common at this stage than other non-REM stages, though not as common as during REM sleep. Like in stage 2, memory and information processing occur during this stage. Sleep walking or talking may also occur.
Sources: UT Southwestern, National Sleep Foundation, American Sleep Association.
Media Contact
James Beltran
[email protected]
214-648-3404
@UTSWNews
http://www.swmed.edu