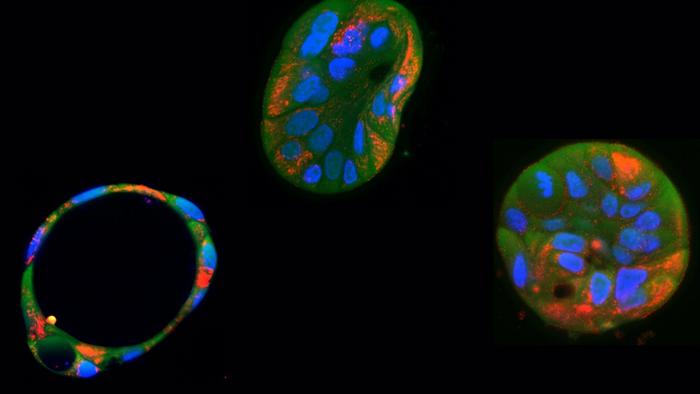
In a groundbreaking advancement that could redefine the future of mitochondrial medicine, scientists from the Netherlands have harnessed the precision of mitochondrial base editing to correct deleterious mutations in human cells. This remarkable achievement, detailed in the open-access journal PLOS Biology on June 24, marks a pivotal step toward treating a broad spectrum of mitochondrial diseases—disorders notoriously difficult to target due to the unique properties of mitochondrial DNA (mtDNA). As mitochondria are essential “powerhouses” of the cell, powering metabolic processes, the ability to directly edit their DNA heralds transformative therapeutic possibilities.
Mitochondrial DNA, distinct from the nuclear genome, resides inside the mitochondrion and is inherited maternally. Its mutations contribute not only to a diverse collection of rare genetic diseases but also have implications in cancer progression and age-related cellular decline. Historically, genome editing technologies such as CRISPR-Cas9 revolutionized nuclear DNA manipulation but fell short when applied to mitochondria owing to their impermeable double membranes and the absence of natural RNA import pathways necessary for CRISPR’s function.
The innovative approach developed by the research team circumvents these challenges by deploying a highly specialized tool known as a mitochondrial base editor. This editor, a double-stranded DNA cytosine base editor (DdCBE), enables precise conversion of cytosine to thymine within the mitochondrial genome without necessitating the formation of double-stranded breaks. This subtle yet powerful mechanism ensures minimal genomic disruption, a crucial advantage given the sensitivity of mitochondrial functions.
.adsslot_Nkps3q6lre{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_Nkps3q6lre{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_Nkps3q6lre{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
In rigorous laboratory experiments, the researchers first engineered liver cell organoids to harbor a mutation within their mitochondrial DNA that severely compromises cellular energy production. These patient-derived liver organoids—three-dimensional tissue cultures closely replicating physiological conditions—served as effective models to study the pathophysiology of mitochondrial diseases. Upon application of the DdCBE base editor, they successfully corrected the mutation, demonstrating restoration of mitochondrial function and energy metabolism within these cells.
Moreover, the team extended their strategy to skin cells obtained from a patient diagnosed with Gitelman-like syndrome, a rare mitochondrial disorder characterized by electrolyte imbalances and neuromuscular symptoms. By targeting and repairing a pathogenic variant within these patient-derived cells, the scientists were able to restore key physiological indicators of healthy mitochondrial function. This achievement not only underscores the therapeutic potential of mitochondrial base editors but also highlights their ability to function in diverse cell types affected by mitochondrial diseases.
An essential aspect of translating this technology into clinical settings is the development of safe and efficient delivery systems for the gene editing components. The researchers innovated by delivering the RNA message encapsulating the base editor’s instructions in the form of messenger RNA (mRNA) rather than DNA plasmids, thereby mitigating the risk of genomic integration and genotoxicity. Encapsulation within lipid nanoparticles (LNPs) further enhanced delivery efficiency and reduced cellular toxicity. LNP-mediated mRNA delivery, already lauded for its success in mRNA vaccines, offers a promising vector for targeted mitochondrial therapies.
The promise of this technique lies not only in its immediate ability to model mitochondrial diseases in vitro but also in its potential as a direct therapeutic intervention. Historically, patients with mitochondrial disorders had limited treatment options, primarily symptomatic management or supportive care. The advent of a tool capable of directly correcting the root genetic causes within mitochondria could transform clinical approaches, possibly leading to cures rather than palliation.
Importantly, this study leveraged clinic-grade techniques and patient-derived organoids, bringing the research closer to clinical application. The use of human cells and organoid models ensures translational relevance and provides a platform for evaluating therapeutic efficacy and safety with unprecedented accuracy. The approach moves the field from theoretical genome editing strategies toward tangible medical innovations.
Despite the promise, challenges remain. Efficient delivery of base editors in vivo—especially to organs predominantly affected by mitochondrial diseases like muscle and brain tissue—requires further refinement. Immune responses, editing efficiency across diverse mitochondrial haplotypes, and long-term effects of editing in post-mitotic cells present hurdles yet to be fully surmounted. Nonetheless, the demonstrated ability to edit mitochondrial DNA with base editors is a monumental leap forward.
The researchers emphasize that their work symbolizes the dawn of a new era in mitochondrial medicine, one where gene editing technologies will finally bridge the long-standing gap presented by mitochondrial genetics. For decades, mitochondrial patients have lagged behind the CRISPR revolution, but innovations such as these offer renewed hope, moving toward therapies that correct mutations at their genetic origin rather than managing their downstream consequences.
As the field anticipates further experimental validation and eventual clinical trials, this discovery stands as a testament to the power of innovative genetic engineering. The convergence of molecular biology, bioengineering, and clinical science is paving the way for novel interventions capable of rewriting the mitochondrial genome, reshaping the landscape of genetic disease treatment forever.
Subject of Research: Cells
Article Title: Correction of pathogenic mitochondrial DNA in patient-derived disease models using mitochondrial base editors
News Publication Date: June 24, 2025
Web References: https://doi.org/10.1371/journal.pbio.3003207
References: Joore IP, Shehata S, Muffels I, Castro-Alpízar J, Jiménez-Curiel E, Nagyova E, et al. (2025) Correction of pathogenic mitochondrial DNA in patient-derived disease models using mitochondrial base editors. PLoS Biol 23(6): e3003207.
Image Credits: Martijn Koppens (CC-BY 4.0)
Keywords: mitochondrial diseases, base editing, mitochondrial DNA, DdCBE, lipid nanoparticles, mRNA delivery, gene therapy, mitochondrial mutations, patient-derived organoids, precision medicine, mitochondrial genome editing, mitochondrial pathology.
Tags: CRISPR technology limitationsgene editinghereditary genetic diseaseshuman cell therapyinnovative biotechnologymetabolic processes in cellsmitochondrial base editingmitochondrial disorders treatmentmitochondrial DNA editingmitochondrial medicinemitochondrial mutationstherapeutic advancements in genetics