Credit: Dr Chester L. Drum
A team from the NUS Yong Loo Lin School of Medicine (NUS Medicine) has invented a fundamentally new way of folding and protecting recombinant proteins. Sourced from the rapidly expanding field of synthetic biology, this protein-in-a-protein technology can improve functional protein yields by 100-fold and protect recombinant proteins from heat, harsh chemicals and proteolysis.
The expression and stabilisation of recombinant proteins is the cornerstone of the biologics and pharmaceutical industries. The costs and complexity associated with manufacturing difficult-to-fold recombinant proteins at an industrial scale are a significant limiting factor to their use in clinical and industrial applications.
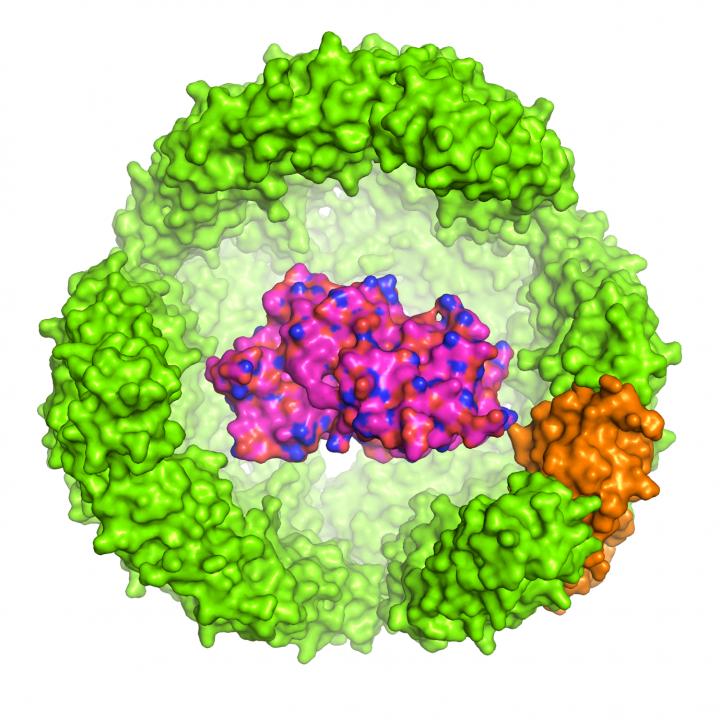
The study led by Dr Chester Drum, Assistant Professor at the Departments of Medicine and Biochemistry, NUS Medicine was published in the journal Nature Communications on 13 November 2017. Dr Drum and colleagues engineered a 12-nanometre diameter exoshell and wrapped it around a protein of interest (POI). They showed for the first time that this technology can be used to fold and protect a variety of proteins within engineered cavities that are less than 1:10,000 the width of a human hair.
The researchers developed this protein-within-a-protein technology with the help of Archeoglobus fulgidus, a hardy bacteria that is naturally found in hydrothermal vents. These hyper-thermophilic bacteria have evolved unique solutions for protein folding and stabilisation due to the extreme environments in which they live.
In particular, the researchers made use of an iron-carrying, 24-subunit protein in A. fulgidus called ferritin, whose natural function is to store and carry iron in the blood. Ferritin from A. fulgidus has two unique properties: first, four tiny pores in its shell provide small molecules access into the cavity; second, unlike human ferritin which is stable at low salt concentrations, the engineered A. fulgidus ferritin dissociates at low salt concentrations, allowing the contents of the cavity to be released by a simple pH switch from 8.0 to 5.8. Once dissociated, the POI can be released enzymatically.
To demonstrate the wide versatility of their technology, the researchers tested their exoshell technology by fusing one of the 24 ferritin subunits around three POIs with diverse properties — green fluorescent protein, horseradish peroxidase (HRP) and Renilla luciferase.
Not only did the exoshell help increase the yields of all three POIs, the researchers were also able to deliver cofactors heme and calcium, in addition to oxidising conditions, to ensure that complex POIs such as HRP protein could fold and function properly.
Besides helping to fold the POIs correctly, the exoshells were also protective against a wide range of denaturants, including high concentration trypsin; organic solvents such as acetonitrile and methanol; and denaturants such as urea, guanidine hydrochloric acid, and heat.
"We hypothesise that the significant increase in functional protein yield may be due to the complementation between the negatively charged proteins and the positively charged exoshell internal surface. Our findings highlight the potential of using highly engineered nanometer-sized shells as a synthetic biology tool to dramatically affect the production and stability of recombinant proteins," said Dr Drum, who is also a consultant cardiologist at the National University Hospital and director of the Clinical Trial Innovation Lab at TLGM, A*STAR.
Recruited to the National University of Singapore in 2011, he has since received funding from the Singapore MIT Alliance for Research and Technology, National Medical Research Council, Biomedical Research Council, A*STAR and NUS Medicine.
Dr Drum's current research bridges the gap between basic biochemistry and clinical care. He is currently the primary investigator on a multi-institutional, 3,000-person observational trial in Singapore that studies how personalised drug metabolism affects drug response.
###
Media Contact
Simin WANG
[email protected]
65-677-27804
http://medicine.nus.edu.sg/corporate/index.html
Related Journal Article
http://dx.doi.org/10.1038/s41467-017-01585-2