In the ever-escalating battle against antibiotic resistance, scientists have honed in on a fresh and compelling target: the bacterial flagellum. This remarkable molecular machine enables bacteria to move, acting as a microscopic propeller that drives infections throughout the body. Unlike traditional antibiotics, which typically seek to eradicate bacteria outright, interfering with the flagellum offers the tantalizing possibility of disarming pathogens without killing them, potentially slowing down the pace at which resistance develops. This novel approach could represent a paradigm shift in how we treat bacterial infections.
The bacterial flagellum is an intricate and highly evolved structure, fundamental to bacterial mobility and pathogenicity. It functions by rotating its long filament, allowing bacteria to “swim” through bodily fluids such as the bloodstream, tissue, and mucus layers. This mobility is critical for bacteria to colonize and infect host cells efficiently. Targeting this motility system could severely impair a bacteria’s ability to cause disease without exerting lethal pressure, which often accelerates antibiotic resistance.
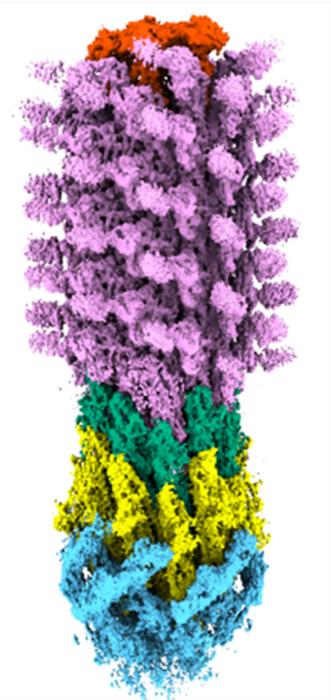
One of the central challenges to attacking the bacterial flagellum lies in our detailed understanding of its structure and assembly—knowledge that until now has been frustratingly incomplete. For over seven decades, the flagellum has captivated researchers worldwide because of its elegant complexity and essential biological function. Yet, despite intense study, the precise three-dimensional atomic architecture of this molecular propeller remained elusive, a mystery locked behind the limitations of past imaging techniques.
.adsslot_Bl6FpetT30{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_Bl6FpetT30{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_Bl6FpetT30{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
The breakthrough came through the use of cryo-electron microscopy (cryo-EM), a revolutionary imaging method that allows scientists to observe cellular structures at near-atomic resolution. This technology involves flash-freezing specimens and imaging them with powerful electron beams, revealing details that conventional microscopy methods cannot achieve. Researchers at King’s College London harnessed one of the most advanced cryo-EM instruments, housed at the Francis Crick Institute, to decode the full architecture of the bacterial flagellum with unprecedented clarity.
By obtaining detailed images revealing the step-by-step assembly of the flagellum’s components, the researchers could identify vulnerable points in its construction line—potential weak spots where new antibiotics could intervene. This molecular choreography of flagellin protein folding and filament growth, previously a “black box” obscured from detailed observation, is now captured like a meticulously shot cinematic sequence of a complex ballet at the atomic scale.
What makes targeting the flagellum particularly attractive is its non-lethal mechanism of action. Conventional antibiotics often work by killing bacteria or inhibiting their replication, applying significant evolutionary pressure on these microorganisms and inevitably selecting for resistant strains. Conversely, disabling the flagellum would incapacitate bacterial mobility and disease-causing capability without necessarily killing the cell. This approach could lessen the selective pressure, potentially curbing the rapid emergence of antibiotic resistance genes.
The public health implications are profound. According to projections by the Global Research on Antimicrobial Resistance Project, drug-resistant infections may claim upward of 39 million lives by 2050 if new interventions and policies are not implemented. The flagellum-targeting strategy offers a promising avenue to mitigate this looming crisis by introducing treatments that thwart infections through novel mechanisms, broadening the arsenal against resistant pathogens.
The detailed insights gained from this study also underscore the importance of interdisciplinary collaboration. Genetic techniques developed at the Max Planck Unit for the Science of Pathogens in Germany enabled the team to isolate and study short segments of the flagellum in isolation, revealing precise insights into flagellin insertion and folding processes. This fusion of cutting-edge microscopy and molecular biology provides a comprehensive understanding that is necessary for rational drug design.
Despite the exciting progress, significant work remains. Researchers still seek to uncover the triggers that initiate flagellum assembly within bacterial cells. Understanding these mechanistic cues could provide additional targets for interference or synergistic therapeutic strategies. Moreover, translating these foundational scientific insights into effective clinical treatments will require sustained funding, rigorous development, and collaboration with pharmaceutical industry partners.
Dr. Julien Bergeron, who led the research at King’s College London, remarked on the transformative potential of their findings. While hopeful that new treatments may emerge within the coming decade, he emphasized the need for ongoing investment and partnerships to realize this promise in the global fight against antimicrobial resistance. The study’s revelations mark a crucial step forward, opening new pathways to develop antibiotics that neutralize bacteria’s disease-causing capacities without fueling resistance evolution.
In sum, the uncovering of the bacterial flagellum’s atomic architecture represents a landmark moment in microbiology and drug discovery. This advance not only deepens scientific understanding of one of nature’s most sophisticated molecular machines but also introduces a practical and potentially revolutionary strategy to combat one of medicine’s most critical challenges. As antibiotic resistance continues to threaten global health, such innovative research illuminates hopeful new directions for treatment development.
Subject of Research: Bacterial flagellum structure and its role as a novel target for antibiotic development to combat antimicrobial resistance.
Article Title: Unraveling the Atomic Architecture of the Bacterial Flagellum: A New Frontier in the Fight Against Antimicrobial Resistance
News Publication Date: Not specified
Web References: Not provided
References: Study published in Nature Microbiology
Image Credits: Dr Julien Bergeron – King’s College London
Keywords: Antibiotic resistance, drug targets, medicinal chemistry, structural biology, cell biology, flagella
Tags: advancements in microbiologyantibiotic resistance strategiesbacterial flagellum as a targetbacterial motility and pathogenicitychallenges in bacterial researchcombating antimicrobial resistancedisarming pathogens without killinginnovative approaches to infection treatmentmolecular machines in bacterianon-lethal antibiotic alternativesparadigm shift in bacterial infection treatmentunderstanding flagellum structure and assembly