Key points:
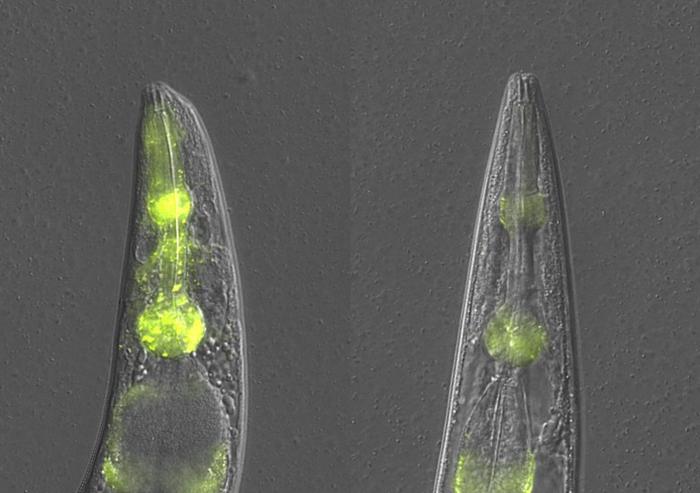
Credit: Raimund Jung (CC-BY 4.0, https://creativecommons.org/licenses/by/4.0/)
Key points:
-
Protein aggregation in certain tissues is a hallmark of diseases such as neurodegeneration and occurs during ageing, but little is known about how protein quality control mechanisms acting to prevent toxic protein build-up vary on a tissue-by-tissue basis.
-
Dr Della David and her team have discovered a safety mechanism that acts to lower levels of protein aggregation in C. elegans pharyngeal muscles, but is not active in body-wall muscles.
-
This new mechanism was identified by comparing protein accumulation in different tissues in ageing nematode worms when normal quality control mechanisms are impaired.
-
By further understanding how some tissues employ protective protein control pathways, the research may help develop future strategies that prevent protein aggregation in vulnerable tissues during ageing.
Researchers from the Babraham Institute, UK, and the German Center for Neurodegenerative Diseases (DZNE) have identified a backup mechanism of protein quality control which prevents the toxic effects of protein aggregation in specific tissues when normal methods of molecular monitoring fail. By understanding how different tissues tackle protein build up, this research could accelerate the identification of ways to protect tissues that are vulnerable to protein build up, possibly tackling both disease-associated protein aggregates and also age-dependent aggregates that accelerate the functional decline of tissues.
Just like factories identifying faulty items coming off the production line, cells use different mechanisms to monitor protein production, folding and accumulation. During ageing some proteins become prone to accumulating due to disrupted protein folding and the decline in the protein quality control mechanisms. Protein clumps called aggregates cause problems for normal functioning of the organism. This increase in protein accumulation is not evenly distributed across the body and some tissues are more likely to accumulate aggregates of certain proteins than others, for example amyloid plaques that build up in the brain during Alzheimer’s disease. What drives the tissue-specific vulnerability or resistance to protein aggregation remains poorly understood.
By studying protein accumulation in the nematode worm C. elegans Dr Della David and her team found that even when the typical protein quality control mechanisms were disrupted, there were lower levels of protein aggregation in the feeding organ of aged worms, the pharynx, compared to the body walls. Their experiments revealed a tissue-specific mechanism they’ve called ‘SAPA: safeguard against protein aggregation’ which kicks in when other protein quality control mechanisms are defective. When activated, this mechanism alleviated proteotoxicity and partially restored pharyngeal function.
“Organisms have a set of control mechanisms found in all tissues that deal with the build-up of defective proteins. In this work we have identified a new tissue-specific control mechanism. This safety mechanism is triggered when conventional control mechanisms are impaired and we’re excited about its discovery because it reveals an extra layer of protection which can be triggered to protect tissues in times of stress, actively stopping protein aggregation and restoring function to the organ” said Dr Della David, group leader in the Signalling research programme at the Babraham Institute.
But how is this specificity achieved? To prevent aggregation specifically in the pharynx,
the safety mechanism relies on a specific and understudied pathway made up of the cells rubbish disposal system called macroautophagy-independent lysosomal degradation. Surprisingly, it also uses factors previously unrelated to managing protein aggregation but known to be involved in the host’s response to natural pathogens that specifically affect the digestive tract.
The team went on to uncover the way that the SAPA mechanism prevents protein accumulation. By closely tracking the production of an aggregating protein and the aggregation dynamics, the team found that the newly discovered mechanism recognises and eliminates newly synthesised proteins before they can form large aggregates.
Dr David summarised, “A big conundrum in our efforts to understand neurodegenerative disease is why particular areas of the brain show aggregates and others don’t. The existence of local protective mechanisms could help explain why some brain areas are more resistant to protein aggregation. More widely, our fundamental research in this area is important to inform therapeutic interventions for diseases of protein aggregation as well as ways to prevent undesirable protein aggregation that occurs with age.”
Journal
PLoS Biology
DOI
10.1371/journal.pbio.3002284
Method of Research
Experimental study
Subject of Research
Animals
Article Title
A safety mechanism enables tissue-specific resistance to protein aggregation during aging in C. elegans
Article Publication Date
14-Sep-2023




