Credit: Kathryn Faith
One of humankind's oldest industrial partners is yeast, a familiar microbe that enabled early societies to brew beer and leaven bread and empowers modern ones to synthesize biofuels and conduct key biomedical research. Yeast remains a vital biological agent, yet our ability to explore and influence its genomic activity has lagged.
In a new article in Nature Communications, University of Illinois researchers describe how their successful integration of several cutting-edge technologies–creation of standardized genetic components, implementation of customizable genome editing tools, and large-scale automation of molecular biology laboratory tasks–will enhance our ability to work with yeast. The results of their new method demonstrate its potential to produce valuable novel strains of yeast for industrial use, as well as to reveal a more sophisticated understanding of the yeast genome.
"The goal of the work was really to develop a genome-scale engineering tool for yeast . . . traditional metabolic engineering focused on just a few genes and the few existing genome-scale engineering tools are only applicable to bacteria, not eukaryotic organisms like yeast," said Steven L. Miller Chair of Chemical and Biomolecular Engineering Huimin Zhao, who led the study. "A second innovation is the use of synthetic biology concepts, the modularization of the parts, and integration with a robotic system, so we can do it in high-throughput."
The team focused on yeast in part because of its important modern-day applications; yeasts are used to convert the sugars of biomass feedstocks into biofuels such as ethanol and industrial chemicals such as lactic acid, or to break down organic pollutants. Because yeast and other fungi, like humans, are eukaryotes, organisms with a compartmentalized cellular structure and complex mechanisms for control of their gene activity, study of yeast genome function is also a key component of biomedical research.
"In basic science, a lot of fundamental eukaryotic biology is studied in yeast," said Tong Si, a Carl R. Woese Institute for Genomic Biology Research Fellow. "People have a limited understanding of these complicated systems. Although there are approximately 6,000 genes in yeast, people probably know less than 1,000 by their functions; all the others, people do not know."
The group took the first step toward their goal of a novel engineering strategy for yeast by creating what is known as a cDNA library: a collection of over 90% of the genes from the genome of baker's yeast (Saccharomyces cerevisiae), arranged within a custom segment of DNA so that each gene will be, in one version, overactive within a yeast cell, and in a second version, reduced in activity.
Zhao and colleagues examined the ability of the CRISPR-Cas system, a set of molecules borrowed from a form of immune system in bacteria (CRISPR stands for clustered regularly interspaced short palindromic repeats, describing a feature of this system in bacterial genomes). This system allowed Zhao to make precise cuts in the yeast genome, into which the standardized genetic parts from their library could insert themselves.
"The first time we did this, in 2013, there was no CRISPR . . . the best we could get was 1% of the cells modified in one run," said Si. "We struggled a little on that, and when CRISPR came out, that worked. We got it to 70% [cells modified], so that was very important."
With gene activity-modulating parts integrating into the genome with such high efficiency, the researchers were able to randomly generate many different strains of yeast, each with its own unique set of modifications. These strains were subjected to artificial selection processes to identify those that had desirable traits, such as the ability to survive exposure to reagents used in the biofuel production process.
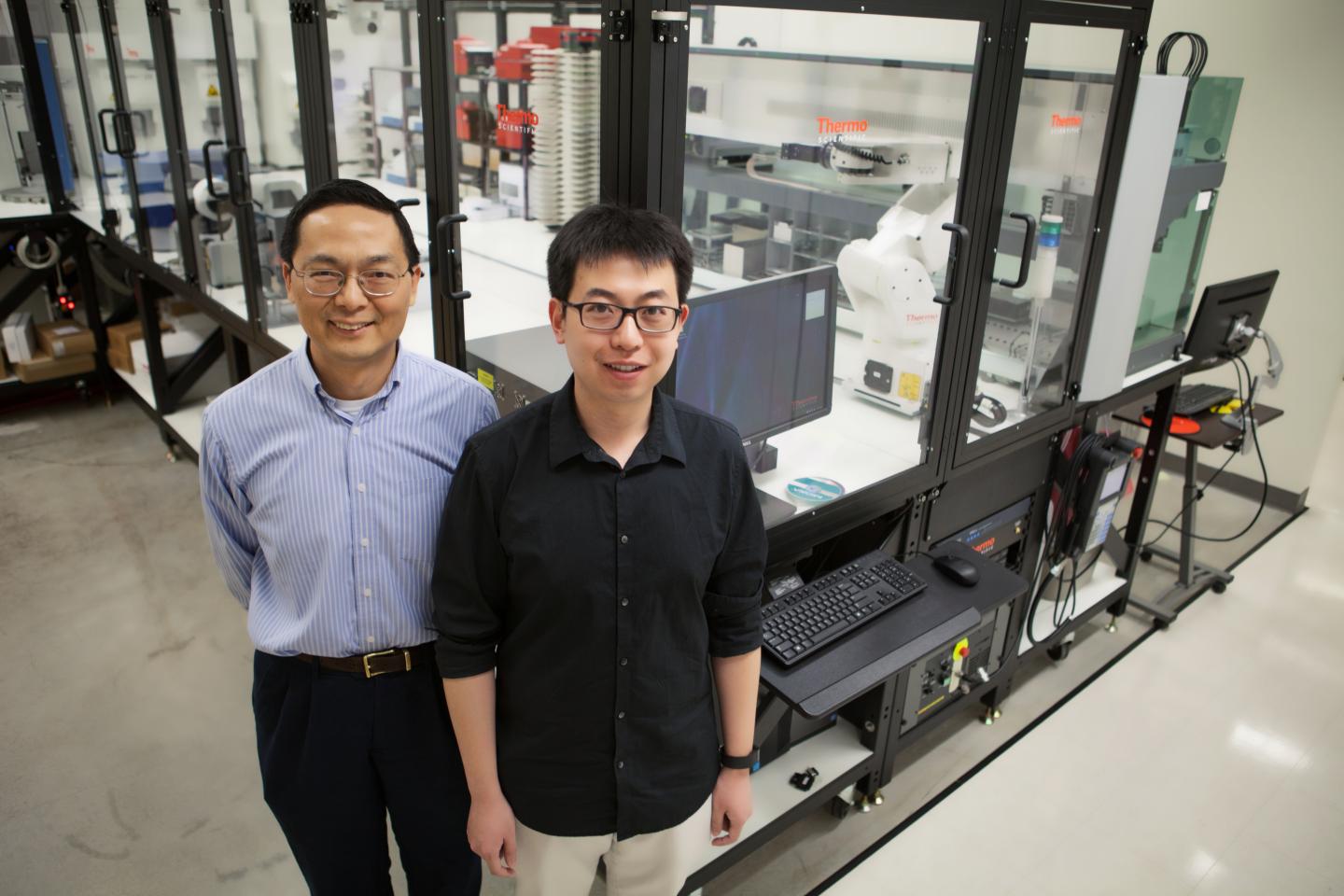
This selection process was greatly aided by the Illinois Biological Foundry for Advanced Biomanufacturing (iBioFAB), a robotic system that performs most of the laboratory work described above in an automated way, including selection of promising yeast strains. Use of iBioFAB greatly accelerated the work, enabling simultaneous creation and testing of many unique strains. The iBioFAB was conceived and developed by the Biosystems Design research theme at the Carl R. Woese Institute for Genomic Biology (IGB), which is led by Zhao.
With support from the High Performance Biological Computing Group at Illinois, Zhao, Si and their colleagues analyzed the modified genomes of their most promising yeast strains. They identified combinations of genes whose altered activities contributed to desirable traits; the functions of some of these genes were previously unknown, demonstrating the technique's ability to generate new biological knowledge.
"I think the key difference between this method and the other existing metabolic engineering strategies in yeast is really the scale," said Zhao. "The current metabolic engineering strategies are all focused on just a few genes, dozens of genes at most . . . it's very intuitive. With this we can explore all the genes, we can identify a lot of targets that cannot be intuited."
###
The work, which was funded by the Roy J. Carver Charitable Trust, IGB, Defense Advanced Research Program Agency, and National Academies Keck Futures Initiative on Synthetic Biology, paves the way for similar approaches to broad-scale, automated genome engineering of other eukaryotic species.
Media Contact
Nicholas Vasi
[email protected]
@IGBIllinois
http://www.igb.uiuc.edu
############
Story Source: Materials provided by Scienmag