Credit: Keiji Mori, TUAT
Every chemical, from the simplest to the most complex, have a structural skeleton of atoms. The atoms can be added or removed to transform the chemical into different types, for use in different applications. For many pharmaceutical and agricultural chemicals, the skeleton consists of a multi-membered carbon ring called a carbocycle.
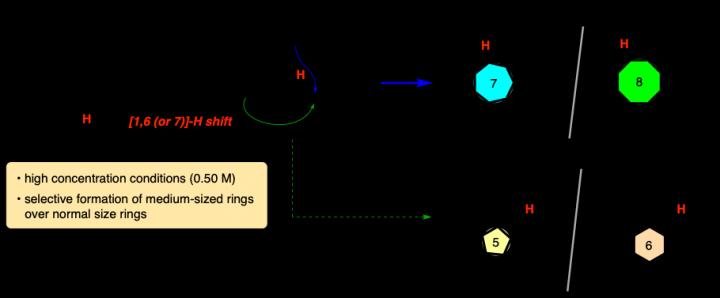
It’s incredibly difficult to make a carbocycle with more than five or six members, but a research team at Tokyo University of Agriculture and Technology (TUAT) in Japan has developed a new method that can easily produce seven- and eight-membered carbocycles.
They published their results in the November 28 print edition of Chemical Communications, a journal of the Royal Society of Chemistry.
“Generally speaking, formation of middle-sized rings is a difficult issue because of their instability and disorder,” said Keiji Mori, paper author and associate professor in the Department of Applied Chemistry in the Graduate School of Engineering at TUAT. “In this study, we developed a simple and effective method for the construction of seven- and eight-membered carbocycles.”
Mori and co-author Yuna Otawa, also with the Department of Applied Chemistry in the Graduate School of Engineering at TUAT, used a process called the “internal redox process.”
The carbocycles include carbon atoms bonded to hydrogen atoms. The system of carbocycles is oxidized, which involves the chemicals rearranging and exchanging components. This weakens the bonds between carbons and hydrogens, allowing hydrogen atoms to bond to a different carbon. The researchers induced this process in a cyclical manner, causing hydrogen shift to distal carbon, leading to a cleanly arranged, medium-sized (seven- and eight-membered) carbocycles. Noteworthy point is that their formation is overwhelmingly favored compared to five- or six-membered ring formation, which are more facile process.
“The construction of seven-membered or larger carbocycles is a major research topic in modern synthetic organic chemistry,” Mori said. “Many organic chemists have devoted much time and effort to the development of an effective method for the synthesis of this class of skeleton, but most of the reported methods require relatively dilute conditions and special precautions to suppress unwanted intermolecular reactions, thereby decreasing the practicality of the synthesis.”
According to Mori, the new method can be performed without special precautions and without undesired molecular effects.
“Our ultimate goal is the development of a synthetic method to two types of medium-sized rings fused-polycycles, which is difficult to achieve with current conventional methods,” Mori said.
###
Contact:
Keiji Mori, PhD
Associate Professor
Department of Applied Chemistry, Graduate School of Engineering, TUAT
[email protected]
For information about the Mori laboratory, please visit http://web.
Original publication:
Yuna Otawa and Keiji Mori
Construction of seven- and eight-membered carbocycles by Lewis acid catalyzed C(sp3)-H bond functionalization.
Chem. Commun., 2019, 55, 13856-13859
About Tokyo University of Agriculture and Technology
Tokyo University of Agriculture and Technology (TUAT) is a distinguished university in Japan dedicated to science and technology. TUAT focuses on agriculture and engineering that form the foundation of industry, and promotes education and research fields that incorporate them. Boasting a history of over 140 years since our founding in 1874, TUAT continues to boldly take on new challenges and steadily promote fields. With high ethics, TUAT fulfills social responsibility in the capacity of transmitting science and technology information towards the construction of a sustainable society where both human beings and nature can thrive in a symbiotic relationship. For more information, please visit http://www.
Media Contact
Yutaka Nibu, Ph.D.
[email protected]
81-423-887-550
Related Journal Article
http://dx.




