Credit: Sun Lab / Seto Lab / Brown University
PROVIDENCE, R.I. [Brown University] –Brown University researchers have developed a new composite catalyst that can perform four separate chemical reactions in sequential order and in one container to produce compounds useful in making a wide range of pharmaceutical products.
"It normally takes multiple catalysts to carry out all of the steps of this reaction," said Chao Yu, a post-doctoral researcher at Brown who co-led the work with graduate student Xuefeng Guo. "But we found a single nanocatalyst that can perform this multistep reaction by itself."
The research, described in the Journal of the American Chemical Society, was a collaboration between the labs of Brown professors Christopher Seto and Shouheng Sun, who are coauthors of the paper.
The work was done, the researchers said, with an eye toward finding ways of making the chemical industry more environmentally sustainable. Multi-reaction catalysts like this one are a step toward that goal.
"If you're running four different reactions separately, then you've got four different steps that require solvents and starting materials, and they each leave behind waste contaminated with byproducts from the reaction," Seto said. "But if you can do it all in one pot, you can use less solvent and reduce waste."
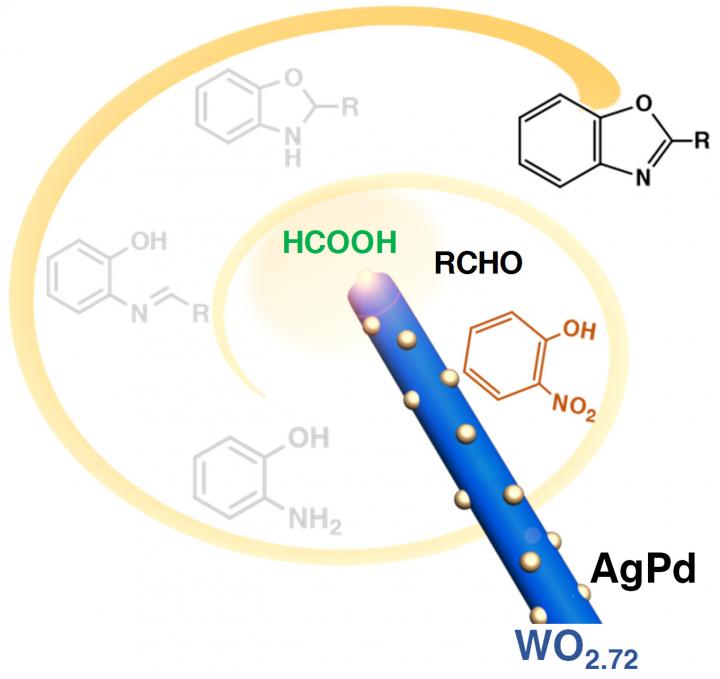
The team made their new catalyst by growing silver-palladium nanoparticles on the surface of nanorods made of oxygen-deficient tungsten-oxide (tungsten-oxide with a few of its oxygen atoms missing). The researchers showed that it could catalyze the series of reactions needed to convert common starting materials formic acid, nitrobenzene and an aldehyde into a benzoxazole, which can be used to make antibacterials, antifungals and NSAID painkillers. The researchers showed that the catalyst could also be used to create another compound, quinazoline, which is used in a variety of anti-cancer drugs.
Experiments showed that the catalyst could perform the four reactions with a nearly quantitative yield — meaning it produces the maximum possible amount of product for a given amount of starting materials. The reactions were performed at a lower temperature, in a shorter amount of time, and using solvents that are more environmentally friendly than those normally used for these reactions.
"The temperature we used to synthesize this product is around 80 degrees Celsius," Guo said. "Normally the reaction happens around 130 degrees and you need to run the reaction for one or two days. But we can get a similar yield at 80 degrees in eight hours."
The new catalyst also is able to make the benzoxazole compounds using starting materials that are more environmentally benign than those generally used. The reaction chain requires a hydrogen source for its initial step. That source could be pure hydrogen gas, which is difficult to store and transport, or it could be extracted from a chemical compound. A compound called ammonia borane is often used for this purpose, but the new catalyst enables formic acid to be used instead, which is "cheaper, greener and less toxic," Yu said.
And while many catalysts tested in these reactions cannot be used more than once without severely damaging their efficiency, the researchers were able to use the new catalyst up to five times with little drop-off in reaction yield.
Sun says that studies like this one represents an emerging line of research in greener chemistry.
"Normally in catalysis we're doing one reaction at a time, with a different catalyst for each reaction" said Shouheng Sun, a professor of chemistry at Brown. "But there's growing interest in coming up with catalysts that can perform multiple reactions in one pot, and that's what we've done here."
###
The work was supported in part by the U.S. Army Research Laboratory and the U.S. Army Research Office (W911NF-15-1-0147).
Media Contact
Kevin Stacey
[email protected]
401-863-3766
@brownuniversity
http://news.brown.edu/
############
Story Source: Materials provided by Scienmag