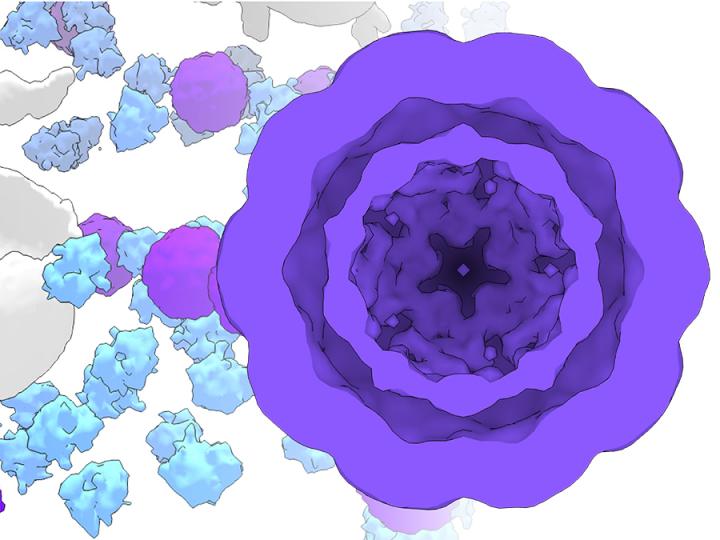
Credit: P. Erdmann / Max-Planck-Institute of Biochemistry
How to install new capabilities in cells without interfering with their metabolic processes? A team from the Technical University of Munich (TUM) and the Helmholtz Zentrum München have altered mammalian cells in such a way that they formed artificial compartments in which sequestered reactions could take place, allowing the detection of cells deep in the tissue and also their manipulation with magnetic fields.
Prof. Gil Westmeyer, Professor of Molecular Imaging at TUM and head of a research team at the Helmholtz Zentrum München, and his team accomplished this by introducing into human cells the genetic information for producing bacterial proteins, so-called encapsulins, which self-assemble into nanospheres. This method enabled the researchers to create small, self-contained spaces – artificial cellular compartments – inside mammalian cells.
Protected areas with new properties
The great strength of the little spheres is that they are non-toxic to the cell and enzymatic reactions can take place inside them without disturbing the cell's metabolic processes. "One of the system's crucial advantages is that we can genetically control which proteins, for example, fluorescent proteins or enzymes, are encapsulated in the interior of the nanospheres," explains Felix Sigmund, the study's first author. "We can thus spatially separate processes and give the cells new properties."
But the nanospheres also have a natural property that is especially important to Westmeyer's team: They can take in iron atoms and process them in such a way that they remain inside the nanospheres without disrupting the cell's processes. This sequestered iron biomineralization makes the particles and also the cells magnetic. "To render cells visible and controllable remotely by making them magnetic is one of our long-term research goals. The iron-incorporating nanocompartments are helping us to take a big step towards this goal," explains Westmeyer.
Magnetic and practical
In particular, this will make it easier to observe cells using different imaging methods: Magnetic cells can also be observed in deep layers with methods that do not damage the tissue, such as Magnetic Resonance Imaging (MRI). In collaboration with Dr. Philipp Erdmann and Prof. Jürgen Plitzko from the Max Planck Institute of Biochemistry, the team could additionally show that the nanospheres are also visible in high-resolution cryo-electron microscopy. This feature makes them useful as gene reporters that can directly mark the cell identity or cell status in electron microscopy, similar to the commonly used fluorescent proteins in light microscopy. Moreover, there are even additional advantages: Cells that are magnetic can be systematically guided with the help of magnetic fields, allowing them to be sorted and separated from other cells.
Use in cell therapy conceivable
One possible future use of the artificial cellular compartments is, for example, cell immunotherapies, where immune cells are genetically modified in such a way that they can selectively destroy a patient's cancer cells. With the new nanocompartments inside the manipulated cells, the cells could in the future be possibly located easier via non-invasive imaging methods. "Using the modularly equipped nanocompartments, we might also be able to give the genetically modified cells new metabolic pathways to make them more efficient and robust," explains Westmeyer. "There are of course many obstacles that have to be overcome in preclinical models first, but the ability to genetically control modular reaction vessels in mammalian cells could be very helpful for these approaches."
###
Publication
Sigmund et al.: „Bacterial encapsulins as orthogonal compartments for mammalian cell engineering", Nature Communications, DOI: 10.1038/s41467-018-04227-3
https://www.nature.com/articles/s41467-018-04227-3
Kontakt:
Prof. Dr. Gil Gregor Westmeyer
Technische Universität München
Professor für Molekulare Bildgebung
Tel.: +49 (0) 89 3187-2123
[email protected]
More Information:
Prof. Gil Gregor Westmeyer is a member of the Munich School of Bioengineering. The study was funded by the ERC Starting grant entitled "MagnetoGenetics" awarded to Gil Gregor Westmeyer.
Short profile of Prof. Gil Gregor Westmeyer http://www.professoren.tum.de/westmeyer-gil/
Munich School of Bioengineering https://www.bioengineering.tum.de/
Website of the research group on "Cell-Circuit-Control" https://www.helmholtz-muenchen.de/ibmi/laboratories/cell-circuit-control/index.html
Media Contact
Vera Siegler
[email protected]
49-892-892-3325
@TU_Muenchen
http://www.tum.de
Original Source
https://www.tum.de/nc/en/about-tum/news/press-releases/detail/article/34644/ http://dx.doi.org/10.1038/s41467-018-04227-3