A comprehensive study conducted by researchers at Tulane University has revealed a notable shift in state-level healthcare legislation aimed at enhancing access to life-saving medications for individuals suffering from opioid use disorder (OUD). This rigorous analysis, published in the esteemed journal Health Affairs, highlights a significant increase in the number of states enacting laws that prohibit private insurance providers from imposing prior authorization requirements for medications critical to opioid addiction treatment. Such legislative measures have surged dramatically over the past decade, reflecting a growing recognition of the opioid epidemic’s severity and the imperative to reduce barriers to effective medical intervention.
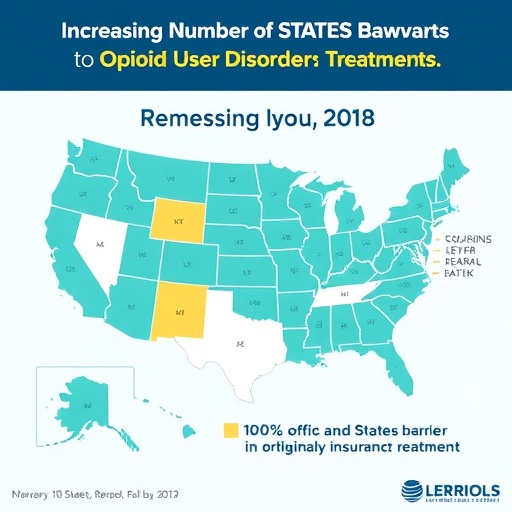
The study meticulously examined statutory changes between 2015 and 2023 to assess the evolving regulatory landscape surrounding prior authorization—a protocol whereby healthcare providers must obtain insurer approval before prescribing certain medications. At the study’s outset in 2015, a mere two states had banned private insurers from requiring prior authorization for medications treating OUD. Fast forward to 2023, and this number has risen dramatically to 22 states, signaling a legislative momentum aimed at dismantling obstacles that delay or prevent timely treatment access.
Fundamentally, prior authorization aims to manage costs and control drug utilization by insurers, but it often results in significant delays for patients requiring urgent therapeutic intervention. These delays can prove devastating for individuals battling opioid dependency, where rapid commencement of medication-assisted treatment (MAT) is pivotal for survival and recovery prospects. The Tulane research team, led by Allison Ju-Chen Hu, an assistant professor of health policy and management, underscores that eliminating prior authorization mandates fosters more expeditious initiation of essential pharmacotherapies, thereby mitigating risks associated with untreated opioid use disorder.
The analysis concentrated specifically on private insurance frameworks, as individuals covered under Medicare or Medicaid typically experience fewer barriers related to prior authorization for OUD medications. Notably, over one-third of people diagnosed with opioid use disorder in the United States have private insurance, rendering this research both timely and crucial for public health policy. The medications at the core of this legislative focus include methadone, buprenorphine, and naltrexone—pharmacological agents that have demonstrated efficacy in curbing opioid cravings, stabilizing neurochemical imbalances, and reducing overdose fatalities.
Importantly, the study details how prior authorization denials compel healthcare providers to seek alternative, often financially burdensome options for their patients. Without insurer approval, patients may face the grim choice of paying out of pocket or forgoing treatment altogether, which exacerbates health disparities and undermines public health objectives aimed at curtailing the opioid crisis. Thus, the legislative prohibition of prior authorization requirements represents a crucial step in harmonizing insurance policies with clinical best practices to improve treatment adherence and outcomes.
The research further reveals a nuanced legislative spectrum: seven states have enacted comprehensive bans on prior authorization for all OUD medications, while 15 have adopted partial bans. These partial restrictions often retain prior authorization for specific drug types or limit prescription durations, though some states, such as New York, Arkansas, Colorado, and Missouri, have subsequently amended their statutes to eradicate these residual barriers entirely. This incremental progress highlights an evolving policy landscape characterized by a blend of cautious experimentation and progressive reform.
In addition to medications directly targeting opioid dependency, the study also identified legislative efforts extending prior authorization prohibitions to naloxone, an opioid antagonist known for its life-saving capacity to reverse overdoses. Eight states have enacted such expansions, coinciding with naloxone’s increased accessibility since its approval for over-the-counter sales in 2023. Although over-the-counter naloxone availability represents a significant public health milestone, insurance coverage remains vital as it substantially lowers out-of-pocket costs and enhances equitable access for vulnerable populations.
The broader context for these regulatory changes is underscored by the alarming statistics of opioid-related mortality. In 2023 alone, approximately 80,000 Americans succumbed to drug overdoses involving opioids—a stark testament to the persistent and escalating toll of the epidemic. Against this grim backdrop, the Tulane study’s findings offer a cautiously optimistic outlook, indicating that legislative interventions aimed at modifying insurance practices could contribute meaningfully to reversing overdose trends by facilitating faster and more reliable access to effective treatments.
Lead author Allison Ju-Chen Hu emphasizes that while legislative bans on prior authorization are promising, their success hinges on robust enforcement mechanisms and insurer compliance. She advocates for future research endeavors to meticulously evaluate the real-world implementation of these laws, focusing on metrics such as delays in treatment initiation, rates of medication adherence, and longitudinal treatment continuity. Such outcomes research is critical to validate the intended public health benefits of these policy changes and to identify potential gaps or unintended consequences.
Moreover, the study encourages exploration into the comparative effectiveness of partial versus full prior authorization prohibitions, given that even limited reforms may serve as foundational steps toward comprehensive policy overhaul. By analyzing diverse legislative approaches, researchers and policymakers can better understand the optimal regulatory frameworks that balance cost containment with patient-centered care imperatives.
In sum, the Tulane University study represents an important contribution to the ongoing discourse on combating the opioid crisis through legal and health policy measures. By illuminating the landscape of insurance-related regulatory innovations and their potential to enhance access to life-saving OUD medications, it supports a data-driven pathway for states to continue strengthening their legislative responses. The interplay between healthcare policy, insurance regulation, and clinical treatment access, as delineated in this research, argues compellingly for continued vigilance, advocacy, and empirical evaluation to ultimately reduce the devastating impact of opioid addiction in the United States.
Subject of Research:
Laws prohibiting prior authorization requirements for opioid use disorder medications in private insurance plans and their implications for treatment access.
Article Title:
State Laws Banning Prior Authorization For Medications For Opioid Use Disorder Increased Substantially, 2015–23
News Publication Date:
3-Nov-2025
Web References:
DOI: 10.1377/hlthaff.2025.00191
Keywords:
Narcotics addiction, methadone, heroin, psychoactive drugs, health insurance
Tags: Health Affairs journal publicationhealthcare access improvementsinsurance barriers to treatmentlegislative changes in addiction treatmentmedications for opioid addictionopiate addiction treatment accessopioid epidemic response strategiesopioid use disorder legislationprior authorization reformprivate insurance regulationsstate healthcare policy changesTulane University research findings