Ammonia (NH3) is one of the most widely produced chemicals in the world, with a production of over 187 million tons in 2020. About 85% of it is used to produce nitrogenous fertilizers, while the rest is used for refining petroleum, manufacturing a wide range of other chemicals, and creating synthetic fibers such as nylon. However, all this comes at a high energy cost. Currently, most of the ammonia is produced using the conventional Haber-Bosch process, which requires combining nitrogen and hydrogen at high temperatures (400-450°C) and pressures (200 atmospheres). As a result, scientists are actively seeking catalysts that can reduce the energy requirements for ammonia production and make the synthesis more sustainable.
Credit: Tokyo Tech
Ammonia (NH3) is one of the most widely produced chemicals in the world, with a production of over 187 million tons in 2020. About 85% of it is used to produce nitrogenous fertilizers, while the rest is used for refining petroleum, manufacturing a wide range of other chemicals, and creating synthetic fibers such as nylon. However, all this comes at a high energy cost. Currently, most of the ammonia is produced using the conventional Haber-Bosch process, which requires combining nitrogen and hydrogen at high temperatures (400-450°C) and pressures (200 atmospheres). As a result, scientists are actively seeking catalysts that can reduce the energy requirements for ammonia production and make the synthesis more sustainable.
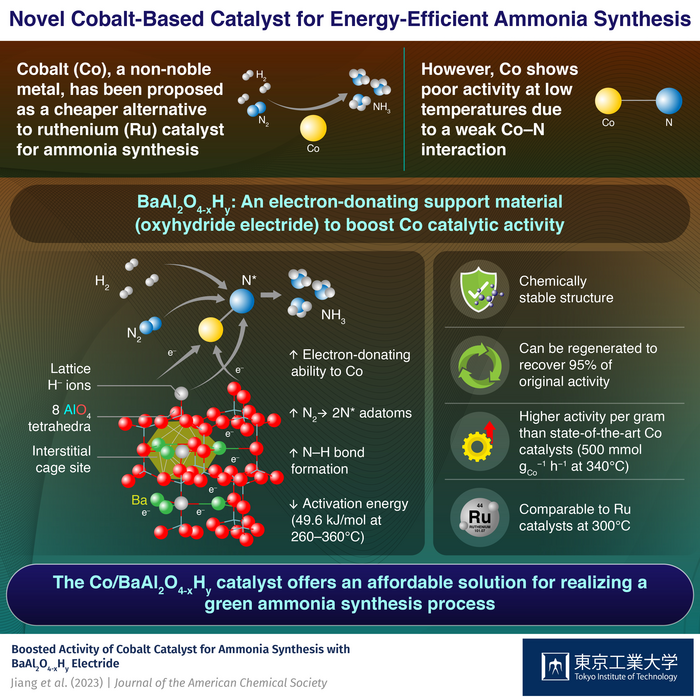
Ruthenium (Ru), a noble metal, has been the primary candidate in this regard owing to its exceptional ability to absorb nitrogen at low temperatures. However, its high cost has prevented its widespread adoption in large-scale ammonia synthesis. While cobalt (Co) has been considered as a more cost-effective alternative, achieving the same catalytic activity as Ru at low temperatures has been difficult.
To enhance the catalytic activity of Co, a team of researchers including Professor Masaaki Kitano at Tokyo Institute of Technology (Tokyo Tech), Japan developed, in a recent study, a support material for Co nanoparticles. The material, a barium-containing oxyhydride electride called BaAl2O4-xHy, increases the catalytic activity of Co to a level comparable to that of Ru catalysts at low temperatures, and protects the H- ions and electrons from the effects of air and moisture. The breakthrough was published in the Journal of the American Chemical Society.
“We attempted to develop a barium-containing oxyhydride electride, Ba2Al2O4–xHy to obtain a highly effective and chemically durable catalyst and unlock a new approach to designing novel inorganic electride materials and triggering their application in other fields,” explains Prof. Kitano.
How did the team achieve this feat? Put simply, BaAl2O4–xHy has a unique structure that promotes the dissociation of nitrogen over Co. The material exhibits a stuffed tridymite structure where AlO4 tetrahedra are linked to form a three-dimensional (3D) network structure, creating cage-like void spaces between the barium ions. These interstitial sites are like pockets for holding negative charges, enabling the material to donate electrons to Co and facilitate the breakdown of nitrogen molecules into nitrogen adatoms.
To improve the electron-donating ability of the material, the researchers introduced electrons to the interstitial sites by replacing the O2- lattice ions with H– ions (O2- (framework)+ ½ H2 = H– (framework) + 1/2 O2 + e– (cage)). The introduction of H– ions not only improved the electron-donating ability of the BaAl2O4 but also facilitated the desired reduction of nitrogen to ammonia.
By promoting both the cleavage of N2 and its subsequent reduction to ammonia, the Co/Ba2Al2O4–xHy catalyst could produce over 500 mmol of ammonia per gram of cobalt per hour, a record value for Co-based catalysts. Moreover, compared to conventional Co catalysts, which typically have activation energies for ammonia synthesis exceeding 100 kJ/mole, the proposed catalyst demonstrated an activation energy of just 48.9 kJ/mole.
Further, the stuffed tridymite structure was durable and reusable, with the AlO4-based tetrahedra framework shielding the lattice H- ions and electrons from oxidation. Finally, after exposing the Co/BaAl2O4–xHy to air, the researchers could recover up to 95% of its original activity by simply heating it in hydrogen.
With its good chemical stability, enhanced catalytic activity, and high reusability, the Co/BaAl2O4–xHy catalyst shows great promise for synthesizing ammonia at low temperatures. “This novel inorganic electride offers a new approach to developing highly effective and stable Ru-free catalysts for green ammonia synthesis,” concludes Prof. Kitano.
Journal
Journal of the American Chemical Society
DOI
10.1021/jacs.3c01074
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Boosted Activity of Cobalt Catalyst for Ammonia Synthesis with BaAl2O4-xHy Electride
Article Publication Date
27-Apr-2023




