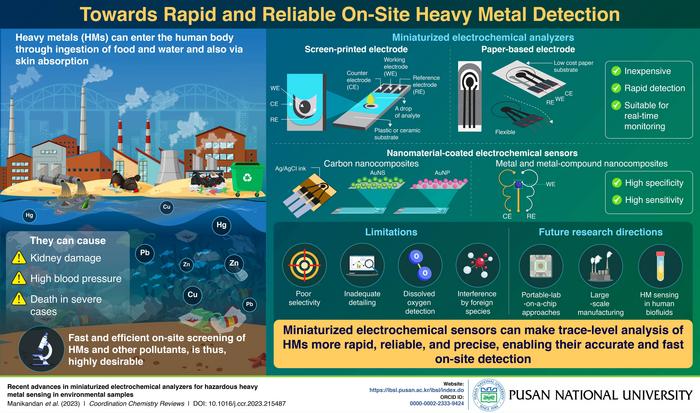
Heavy metals (HMs) are metals with high densities and atomic weights. Originating from geological processes or human activities, including mining, industrial production, and petrochemical plants, they are toxic to humans and animals and considered as common pollutants in the environment. HMs can enter the human body on ingestion of polluted food or water, adsorption through the skin, or respiration of polluted air. They are known to cause severe health problems in humans, such as kidney damage, high blood pressure, nervous system damage, fertility defects, and even death. Hence, precise and compact HM detection technologies are essential to assess their concentrations in the environment and screen for health issues arising from their pollution. To this end, recent years have seen a rise in the use of electrochemical sensing techniques for on-site screening of HM pollutants.
Credit: Seung-Cheol Chang from Pusan National University
Heavy metals (HMs) are metals with high densities and atomic weights. Originating from geological processes or human activities, including mining, industrial production, and petrochemical plants, they are toxic to humans and animals and considered as common pollutants in the environment. HMs can enter the human body on ingestion of polluted food or water, adsorption through the skin, or respiration of polluted air. They are known to cause severe health problems in humans, such as kidney damage, high blood pressure, nervous system damage, fertility defects, and even death. Hence, precise and compact HM detection technologies are essential to assess their concentrations in the environment and screen for health issues arising from their pollution. To this end, recent years have seen a rise in the use of electrochemical sensing techniques for on-site screening of HM pollutants.
In a new study now, a team of researchers from Korea, led by Professor Seung-Cheol Chang from the Department of Optics and Mechatronics Engineering at the College of Nanoscience and Nanotechnology at Pusan National University, comprehensively reviewed the recent developments in electrochemical sensors for heavy metal detection. “Conventional analytical techniques for HM detection are difficult to use for on-field analysis. There is, therefore, an urgent need for portable electrochemical sensors that are easy to use, cost-effective, and suitable for rapid on-site detection,” explains Prof. Chang. First author Dr. Ramalingam Manikandan from Prof. Chang’s lab contributed significantly with a lot of practical work for this study, which was made available online on October 27, 2023, and published in Volume 499 of the journal Coordination Chemistry Reviews on January 15, 2024.
In this review, the team focused exclusively on miniaturized electrochemical sensors that are suitable for on-site detection of HM pollutants. They investigated different sensor variants such as screen-printed electrodes (SPEs), paper-based electrodes, and nanomaterial-coated sensors made from carbon nanocomposites, metal nanoparticles, and metal-compound nanocomposites.
Their analysis revealed that miniaturized electrochemical sensors based on SPEs and paper-based electrodes offer low-cost and time-efficient analysis while also reducing the required amount of sample and supporting electrolytes. These sensors also effectively address the limitations of conventional laboratory-based methods. Additionally, nanomaterial-based sensors exhibit high specificity and sensitivity, enabling the detection of ultra-trace amounts of HMs with high accuracy, in a wide variety of environmental conditions.
Despite these advances, however, the team acknowledged the existing limitations of electrochemical sensors that still need to be addressed. Current electrochemical detection approaches suffer from poor selectivity, inadequate level of detail, and interference by foreign species that can have detrimental effects during on-site analysis. Also, additional encounters with dissolved oxygen species, while necessary for analyzing conductivity and pH, contribute to a decline in the detection ability of these sensors over time.
The researchers also stressed the need for portable lab-on-a-chip approaches and large-scale manufacturing of disposable, flexible, and wearable electrochemical sensors. Moreover, innovative electrochemical detection strategies are required for HM sensing in human biofluid samples, such as saliva, blood, and urine. “One of the most difficult tasks is the commercialization of the advanced and systematic ideas put forward by academia, pharmaceutical industries, and government bodies in combination with proper validation techniques,” says Prof. Chang, while talking about the future of research on electrochemical sensors for HMs.
Nonetheless, the team is confident that ongoing research in electronics, nanotechnology, and materials technology can overcome some of the existing issues, paving the way for more rapid, reliable, and precise on-site detection of HMs for a safer and healthier environment.
In essence, this study sheds light on the current advances in electrochemical detection technologies. These insights can not only serve as a valuable resource for the present but also for inspire future research!
***
Reference
DOI: https://doi.org/10.1016/j.ccr.2023.215487
About the institute
Pusan National University, located in Busan, South Korea, was founded in 1946 and is now the No. 1 national university of South Korea in research and educational competency. The multi-campus university also has other smaller campuses in Yangsan, Miryang, and Ami. The university prides itself on the principles of truth, freedom, and service, and has approximately 30,000 students, 1200 professors, and 750 faculty members. The university is composed of 14 colleges (schools) and one independent division, with 103 departments in all. Website: https://www.pusan.ac.kr/eng/Main.do
About the author
Seung-Cheol Chang is currently a Professor at the Department of Optics and Mechatronics Engineering at Pusan National University, Korea. In 2000, he received his Ph.D. in Clinical Biochemistry from Newcastle University in England. Before coming to Pusan National University, he conducted research on biosensors at Calum McNeil’s lab at Newcastle University Medical School for 7 years. His research group is currently conducting research on the development of bio-mimic nanocomposites-based electrochemical biosensors for environmental or clinical analysis.
Lab website address: https://ibsl.pusan.ac.kr/ibsl/index.do
ORCID id: http://orcid.org/0000-0002-2333-9424
Journal
Coordination Chemistry Reviews
DOI
10.1016/j.ccr.2023.215487
Method of Research
Literature review
Subject of Research
Not applicable
Article Title
Recent advances in miniaturized electrochemical analyzers for hazardous heavy metal sensing in environmental samples
Article Publication Date
27-Oct-2023
COI Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.



