Reston, VA—Detailed PSMA PET mapping of cancer recurrence in the prostate bed shows that current radiotherapy contouring guidelines—which determine the target areas for treatment—miss a significant number of lesions and may irradiate healthy tissues unnecessarily. In a new study published online by The Journal of Nuclear Medicine, researchers are calling for the redefinition of prostate bed contouring guidelines to improve outcomes for patients.
Credit: Image created by Minsong Cao, Dylan O’Connell, and Ida Sonni, University of California Los Angeles, Los Angeles, California.
Reston, VA—Detailed PSMA PET mapping of cancer recurrence in the prostate bed shows that current radiotherapy contouring guidelines—which determine the target areas for treatment—miss a significant number of lesions and may irradiate healthy tissues unnecessarily. In a new study published online by The Journal of Nuclear Medicine, researchers are calling for the redefinition of prostate bed contouring guidelines to improve outcomes for patients.
Approximately one-third of prostate cancer patients who undergo radical prostatectomy experience disease progression within 10 years. Salvage radiation therapy (SRT) is a potentially curative treatment option for these patients. SRT currently follows contouring guidelines based on expert consensus and does not take advantage of the information available from novel imaging techniques, such as PSMA PET.
“PSMA PET is one of the most accurate methods for detecting tumor recurrence after prostatectomy and, since its FDA approval in December 2020, has increasingly changed patterns of care for prostate cancer patients,” said Alan Dal Pra, MD, associate professor and director of clinical research in the Department of Radiation Oncology at the University of Miami Miller School of Medicine in Miami, Florida. “In our study, we sought to analyze the patterns of prostate bed recurrence with PSMA PET and see how they compared to current expert consensus contouring guidelines from the Radiation Therapy Oncology Group (RTOG).”
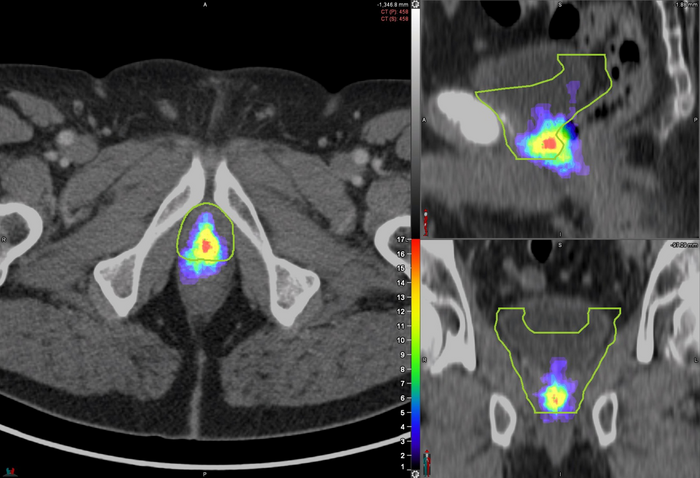
Researchers used PSMA PET to image the patterns of prostate bed recurrence in 127 prostate cancer patients experiencing PSA persistence or biochemical recurrence after radical prostatectomy. For each PSMA PET scan, researchers assessed the location of recurrent lesions and determined the clinical target volume (CTV) based on the RTOG contouring guidelines. The lesions were then categorized as completely covered, partially covered, or not covered by the contouring guidelines.
The recurrent lesions were completely covered in the CTV in 53 percent of patients, partially covered in 34 percent of patients, and not covered in 13 percent of patients. In addition, some areas in the CTV had no evidence of lesions at all.
“We hope this study will help redefine prostate bed contouring guidelines for SRT to improve the outcomes of patients receiving radiotherapy post-radical surgery,” stated Ida Sonni, MD, nuclear medicine physician and academic researcher in the Department of Radiological Sciences at the University of California, Los Angeles in Los Angeles, California. “Our work confirms the crucial and growing role that nuclear medicine and molecular imaging have in guiding decision-making for cancer treatment. Nuclear medicine plays an essential part in the multidisciplinary management of patients with prostate cancer and facilitates the use of individualized, tailored treatments, which ultimately benefit all our patients.”
This study was made available online in February 2023.
The authors of “PSMA PET/CT–based Atlas for Prostatic Bed Recurrence after Radical Prostatectomy: Clinical Implications for Salvage Radiation Therapy Contouring Guidelines” include Ida Sonni, Ahmanson Translational Theranostics Division, Department of Molecular and Medical Pharmacology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California, Department of Radiology, David Geffen School of Medicine, University of California, Los Angeles, California, and Department of Experimental and Clinical Medicine, Nuclear Medicine Unit, Magna Graecia University, Catanzaro, Italy; Alan Dal Pra, Department of Radiation Oncology, University of Miami Miller School of Medicine, Miami, Florida; Dylan P. O’Connell, Stephanie M. Yoon, Jie Deng, Clayton Smith, Minsong Cao, and Amar U. Kishan, Department of Radiation Oncology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California; Zachary Ells, Kathleen Nguyen, and Jeremie Calais, Ahmanson Translational Theranostics Division, Department of Molecular and Medical Pharmacology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Matthias Benz, Ahmanson Translational Theranostics Division, Department of Molecular and Medical Pharmacology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California and Department of Radiology, David Geffen School of Medicine, University of California, Los Angeles, California; Tristan Grogan, Department of Medicine Statistics Core, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; and Nickolas G. Nickols, Department of Radiation Oncology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California and Department of Radiation Oncology, Veteran Affairs Greater Los Angeles Healthcare System, Los Angeles, California.
Visit the JNM website for the latest research, and follow our new Twitter and Facebook pages @JournalofNucMed or follow us on LinkedIn.
###
Please visit the SNMMI Media Center for more information about molecular imaging and precision imaging. To schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or [email protected].
About JNM and the Society of Nuclear Medicine and Molecular Imaging
The Journal of Nuclear Medicine (JNM) is the world’s leading nuclear medicine, molecular imaging and theranostics journal, accessed 15 million times each year by practitioners around the globe, providing them with the information they need to advance this rapidly expanding field. Current and past issues of The Journal of Nuclear Medicine can be found online at http://jnm.snmjournals.org.
JNM is published by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging—precision medicine that allows diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes. For more information, visit www.snmmi.org.
Journal
Journal of Nuclear Medicine
DOI
10.2967/jnumed.122.265025
Article Title
PSMA PET/CT–based Atlas for Prostatic Bed Recurrence after Radical Prostatectomy: Clinical Implications for Salvage Radiation Therapy Contouring Guidelines
Article Publication Date
9-Feb-2023
COI Statement
Alan Dal Pra reports research support to institution/sponsor – Veracyte, outside of the submitted work. Advisory board membership – Merck, outside of the submitted work. Nicholas Nickols reports research support from Lantheus, Janssen, Bayer, outside the submitted work, and consulting for PrimeFour outside the submitted work. Amar U. Kishan reports funding support from grant P50CA09213 from the Prostate Cancer National Institutes of Health Specialized Programs of Research Excellence, grant W81XWH-22- 1-0044 from the Department of Defense, grant RSD1836 from the Radiological Society of North America, the STOP Cancer Organization, the Jonsson Comprehensive Cancer Center, and the Prostate Cancer Foundation; personal fees from Varian Medical Systems, Inc., ViewRay Inc., and Intelligent Automation, Inc.; and research support from ViewRay, Inc., the American Society for Radiation Oncology (ASTRO), the Prostate Cancer Foundation, and the Jonsson Comprehensive Cancer Center, all outside the submitted work. Jeremie Calais reports prior consulting activities for Advanced Accelerator Applications, Astellas, Blue Earth Diagnostics, Curium Pharma, DS Pharma, EXINI, GE Healthcare, Isoray, IBA RadioPharma, Janssen Pharmaceuticals, Lightpointmedical, Lantheus, Monrol, Novartis, Progenics, POINT biopharma, Radiomedix, Sanofi, Telix Pharmaceuticals, outside of the submitted work.




